Harvard Stem Cell Institute Kidney Diseases Program Leader Benjamin Humphreys has examined tissue regeneration in the kidney. His interest in kidney regeneration has occupied a major part of his career, but some of his more recent work resulted from his skepticism of a particular theory of kidney regeneration.
The kidney stem cell repair model postulates that scattered throughout the kidney are small stem cell populations and are activated after the kidney is injured to repair it. This theory, however, conflicts with another view of kidney regeneration. Namely that after injury, the cells of the kidney dedifferentiate into more primordial versions of themselves and proliferate, after which they differentiate into the various tissues of the kidney.
Humphreys and his colleagues now have evidence that strongly suggests that all the cells of the kidney have the capacity to divide after injury and contribute to kidney regeneration.
Their evidence comes in the form of experiments in mice in which the cells of the kidney were genetically tagged, and then the kidneys were injured to determine what cells contributed to the regeneration of the kidney.
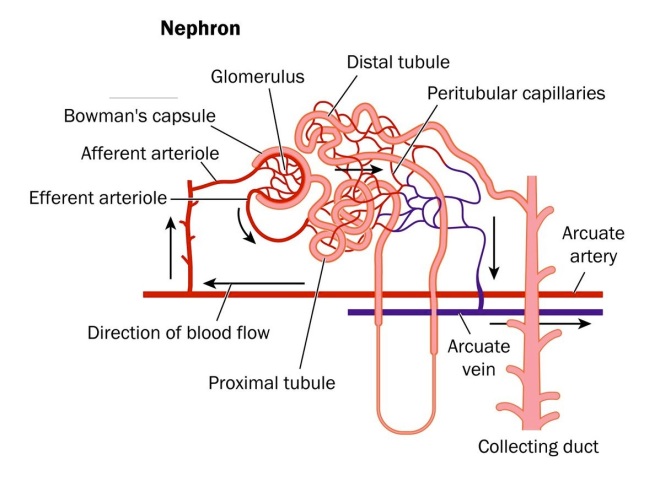
The tagging in this experiment is complicated, but quite technically brilliant. The kidney is composed of myriads of tiny functional units called nephrons. Each nephron is fed by a tiny knot of blood vessels called a glomerulus. The structure of a nephron is shown below.
The blood supply to the kidney comes from branches off the descending aorta knows as renal arteries. After entering the kidneys, the renal arteries branch multiple times until they become tiny vessels that feed into each nephron known as afferent arterioles. The afferent arterioles forms a dense network of knot-like vessels that form the glomerulus and the portion of the nephron that interacts with the glomerulus is known as the Bowman’s capsule.. The blood vessels of the glomerulus are very special because they are exceptionally porous. However, the Bowman’s capsule has a series of cells with foot-like extensions that coat the glomerulus called “podocytes.” An especially beautiful picture of podocytes wrapped around a glomerular vessel is shown below.
The podocytes cover the pores of the glomerulus and only allows water and things dissolved in water through the pores. Proteins do not make it through – they are too heavily charged. Cells also do not make it through – they are too big. But water, sodium ions, potassium ions, hydrogen ions, some drugs, metabolites, waste products, and things like that all make it through the podocyte-guarded pores. For this reason, if you have excessive protein or some blood cells in your urine, it is usually an indication that something is wrong.
Now, rest of the tubing attached to the nephron serve to reabsorb all the things you do not want to get rid of and not absorb all the things you do want to get rid of. The amount of water you eliminate depends on your degree of hydration and is controlled by a hormone called antidiuretic hormone, which is release by the posterior lobe of your pituitary gland when you are dehydrated. In the presence of ADH, the posterior tubing reabsorbs more water, and in lower concentrations of this hormone, it reabsorbs far less.
Now that we know something about the kidney, here’s how Humphreys and others genetically marked the kidneys of their mice. The sodium-dependent inorganic phosphate transporter (SLC34a1) is only expressed in mature proximal tubule cells. Tetsuro Kusaba, the first author on this paper, and his colleagues inserted a CreERT2 cassette into this gene. If you are lost at this point all you need to remember is this: the CreERT2 cassette is inserted into a gene that is ONLY expressed in specific kidney cells. The Cre gene encodes a recombinase that clips out specific bits of DNA from a chromosome. Kusaba and others crossed these engineered mice with another strain of mice that had the gene for a bright red dye inserted into another gene, but this dye could not be expressed because another piece of DNA was in the way. When these hybrid mice were fed a drug called tamoxifen, it activated the expression of the Cre protein, but only in the proximal tubule cells of the kidney and this Cre protein clipped out the piece of DNA that was preventing the red dye gene from being expressed. Therefore, these mice had a particular part of their nephrons, the proximal tubules glowing bright red. This is a stroke of shear genius and it genetically marks these cells specifically and strongly.
Next, Kusaba and colleagues used unilateral ischemia reperfusion injury (IRI) to damage the kidneys. In IRI, the blood supply is stopped to one kidney but not the other for a short period of time (26 minutes). This causes cell death and kidney damage. The other kidney is not damaged and serves as a control for the experiment.
Examination of the damaged kidneys showed that red-glowing cells were found in areas other than the proximal tubules. The only way these cells could have ended up in these places was if the differentiated cells divided and helped repair the damaged parts of the nephrons.
Other research groups have seen similar results, but interpreted them as evidence of stem cell populations in the kidney. However, Humphreys groups discovered something even more fascinating. These “stem cell-markers” in the kidney are actually markers of kidney damage and regeneration and all cells in the kidney express them. In Humphreys words, “What was really interesting is when we looked at the appearance and expression patterns of these differentiated cells, we found that they expressed the exact same ‘stem cell markers’ that these other groups claimed to find in their stem cell populations. And so, if a differentiated cell is able to express a ‘stem cell marker’ after injury, then what our work shows is that that’s an injury marker – is doesn’t define a stem cell.”
Indeed, several genes that have been taken to be signs of a kidney stem cell population (CD133, CD24, vimentin, and KIM-1) were expressed in red-glowing cells. A stem cell population should not be fully differentiated and therefore, should not be able to express the red dye. However, red-glowing cells clearly expressed these found genes after injury. This rather definitely shows that it is the fully differentiated cells that are doing the regeneration in the kidney and not a resident stem cell population. This does not prove that there is no resident stem cell population in the kidney, but only that the lion’s share of the regeneration is done by differentiated cells, and that under these conditions, no stem cell population was detected.
This new interpretation of kidney repair suggests that cells can reprogram themselves in a way that resembles the way mature cells are chemically manipulated to revert to an induced pluripotent state.
See Tetsuro Kusaba, Matthew Lalli, Rafael Kramann, Akio Kobayashi, and Benjamin D. Humphreys. Differentiated kidney epithelial cells repair injured proximal tubule. PNAS (October 14, 2013); doi:10.1073/pnas.1310653110.