New research published in the journal Science Translational Medicine suggests that gene therapy treatments for inherited types of deafness might one day become a reality. This new report shows that fixing faulty DNA sequence in deaf mice can improve their auditory responses. In separate experiments, the drug-maker Novartis is testing a different form of gene therapy in people who have lost their hearing through damage or disease.
Safety missteps in the late 1990s and early 2000s set gene therapy research back several years. During those darker days, gene therapy scientists re-tooled and re-examined their basic assumptions about gene therapy. Even though gene therapy experiments were relatively successful in laboratory mice, humans are not mice, and a whole new set of gene therapy strategies were needed. Fortunately, as a result of this intense research, gene therapy is enjoying a modern-day renaissance. Positive clinical results were observed in clinical trials in 2013 with patients with a blood cancer called acute lymphocytic leukemia, or ALL, and last year in patients an inherited form of blindness called choroideremia.
“We are somewhat late in the auditory field but I think we are getting there now,” said Tobias Moser of the University Medical Center Gottingen, Germany, who was not involved in the new research. “It’s an exciting time for gene therapy in hearing.”
Currently, there are no approved disease-modifying treatments for disabling hearing loss; a condition that affects 360 million people, or 5 percent of the world’s population, according to the World Health Organization. Hearing aids can amplify sounds, and cochlear implants convert sounds into electrical signals for the brain to decode, but such devices cannot fully replicate natural hearing.
The vast majority of hearing loss in older people (known as presbycusis) is noise-induced or age-related, but at least half of deafness that occurs before a baby learns to speak is caused by defects in one of more than 70 individual genes. These are the infants Swiss and U.S. researchers hope to help, after showing that replacing a mutated gene improved the function of hair cells of the inner ear and partially restored hearing in deaf mice.
Scientists from the Ecole Polytechnique Federale de Lausanne and the Boston Children’s Hospital tested hearing in newborn mutant mice by seeing how high they jumped when startled by a noise (startle response). Next, this team focused on a gene called Tmc1. Mutations in Tmc1 commonly cause human genetic deafness, and accounting for 4 to 8 percent of cases of inherited human deafness. But other forms of hereditary deafness can also potentially be “fixed” using the same strategy.
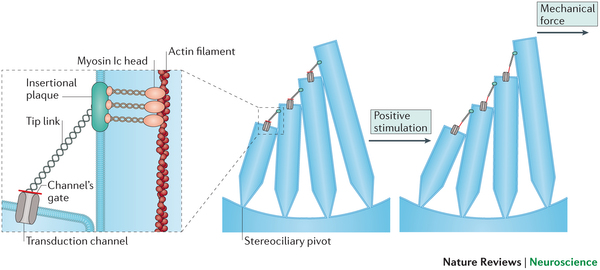
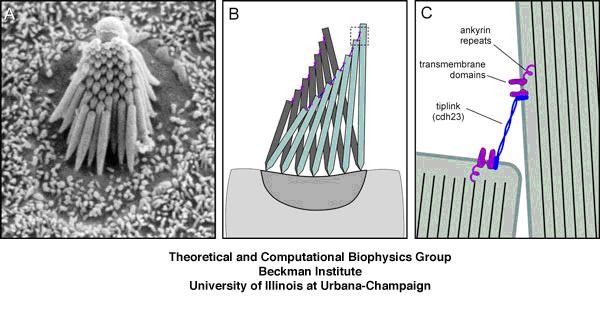
For those who are interested, the Tmc1 gene encodes a protein called Transmembrane channel-like protein 1 (TMC1), which is a membrane-embedded protein that is in the plasma membrane of Hair cells in the cochlea of the inner ear. TMC1 works with another membrane protein called TMC2 to interact with a protein complex called the “Tip link” proteins.
These Tip link proteins, protocadherin 15 and cadherin 23 help TMC1 and TMC2 to transmit signaling into the hair cell when it is deformed by sound waves in the cochlea. Without TMC1, the movements of the hair cells fail to generate any signal within it, and without internal signals, the hair cell will not send any signals to the auditory nerve.
Jeffery Holt and his colleagues used a small virus called adeno-associated virus (AAV) and genetically modified it so that it would carry the TMC1 gene. Next, they found that by using the promoter of the chicken β-actin gene, the TMC1 gene would be highly expressed in cochlear hair cells. When the inner ears of mice were infected with the TMC1-carrying AAVs, the deaf mice showed restored sensory transduction, auditory brainstem responses, and acoustic startle reflexes. This suggest that gene therapy with Tmc1 is well suited for further development as a treatment of auditory function in deaf patients who carry Tmc1 mutations.
Jeffrey Holt of Boston Children’s said their technique still needed work to perfect it, but he is very hopeful that clinical trials in human patients will start within five to 10 years.
Work at Novartis is more advanced, with the first patient treated last October in an early-stage clinical trial that will recruit 45 people in the United States, with results due in 2017. The Swiss company’s product, acquired in a 2010 deal with GenVec worth up to $214 million, delivers a gene called Atoh1 that acts as a master switch for turning on the growth of inner ear hair cells that are central to hearing.