A new report from Johns Hopkins University researchers indicates that a particular stem cells that helps build mouse hearts can self-renew. This discovery, which might very well apply to humans as well, could potentially open inroads to use these cells to repair hearts damaged by disease, or, perhaps, even grow new heart tissue for transplantation.
This study is slated for publication in the journal eLife. Chulan Kwon, Ph.D., an assistant professor of cardiology and member of the Institute for Cell Engineering at the Johns Hopkins University School of Medicine and his team, found that during heart formation, these so-called cardiac progenitor cells or CPCs proliferate, but do not differentiate into heart cells in an embryonic structure known as the second pharyngeal arch. This insight into the biology of CPCs may contribute to better understanding of how to prevent and treat congenital heart defects.
Kwon noted that, “Our finding that CPCs are self-renewing—that they can keep dividing to form new CPCs—means they might eventually be maintained in a dish and used to make specific types of heart cells.”
Kwon continued: “Growing such cells in a dish would be an enormous step toward better treatment for heart disease.”
Kwon’s laboratory initially tackled the elucidating the contribution of two genes, Numb and Numbl, in the CPC biology. Other studies have shown that these two genes are required to guide stem and progenitor cells to their fully mature, specialized functions. Numb and Numbl are highly conserved in mice and humans (i.e. the proteins encoded by these genes in mice and humans are nearly identical). This conservation indicates that Numb and Numbl are probably doing something very important the lives of CPCs.
As a first step, Kwon and others made loss-function mutations in Numb and Numbl. The results were striking. According to Kwon, embryos that lacked functional Numb and Numbl protein, “failed to develop normal hearts and died at an early stage of development, showing us that Numb and Numbl are needed for CPCs to build the heart.”
With the crucial role of Numb and Numbl in the lives of CPCs in mind, Kwon and his colleagues tried to determine the location of CPCs in the developing embryo. For these experiments, they used mouse embryonic stem cells that lacked functional Numb and Numbl, and expressed a glowing red protein in all CPC cells. Such a glowing red protein would instantly give away the CPCs’ location. These embryonic stem cells have the ability to integrate into a growing embryo, but the absence of functional Numb and Numble proteins in these cells prevents them from growing into a viable embryo.
Next, Kwon’s group injected these engineered embryonic stem cells into viable mouse embryos at the blastocysts stage. The blastocyst stage forms early during mammalian development, and it consists of the two cell populations that will form the embryo (inner cell mass cells) and the placenta (trophoblast cells). “The normal cells in these blastocysts compensated for those that lacked Numb and Numbl, allowing the resulting embryos to survive,” Kwon says.
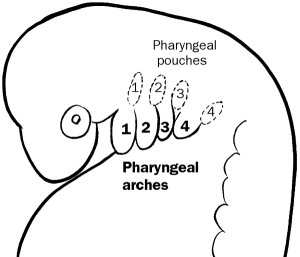
Once these chimeric embryos began to grow, Kwon’s group examined them for red-glowing cells. They found the glowing red cells in the second pharyngeal arch, which is known for forming parts of the neck and face. Kwon says their study is the first to identify the second pharyngeal arch as the home of the CPCs.
The cells of the second pharyngeal arch go on to form the stapes in the middle ear and the stapedius muscle that attaches to the stapes.
Additionally, Kwon’s group cultured second pharyngeal arch cells with CPCs. They discovered that the cultured CPCs self-renewed without developing into specialized heart cells. This is potentially an important step toward using CPCs to treat heart disease.
The next step, he says, is to direct the laboratory-grown CPCs to form new heart tissue that could be used to regenerate disease-damaged heart tissue. “Eventually, we might even be able to deliver cells to damaged hearts to repair heart disease,” Kwon says.