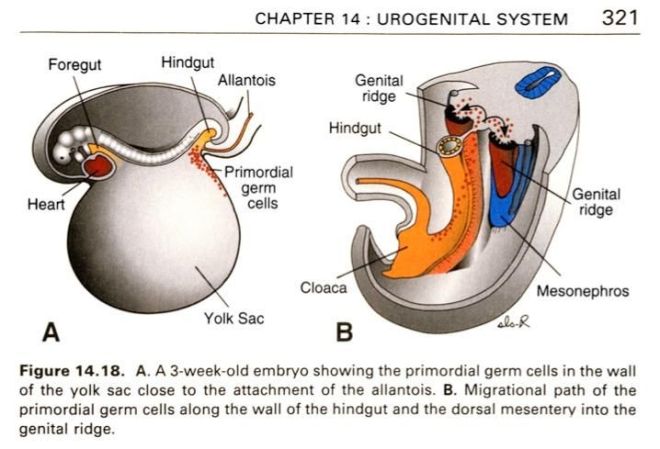
Eduardo Marbán from Cedars-Sinai Heart Institute in Los Angeles and his team have invested a great deal of work into the development of cardiospheres, which are self-assembling heart-derived stem cells that grow as little balls of cells in culture. Several preclinical experiments and a few clinical trials have established the effectiveness of cardiospheres are treatments for the heart after a heart attack. However, Marbán and his co-workers have also worked very hard at determining why cardiospheres heal a damaged heart.
To that end, Marbán and others have returned to their mouse model to do very detailed experiments with their cardiospheres and define exactly why these cells help heal the heart. To date, it is clear that cardiospheres increase the density of blood vessels in the heart tissue, decrease scar deposition, and prevent heart remodeling (the enlargement of the heart after a heart attack to compensate for the increase load placed on smaller amount of heart tissue). Marbán and others wanted to know precisely how cardiospheres managed these feats.
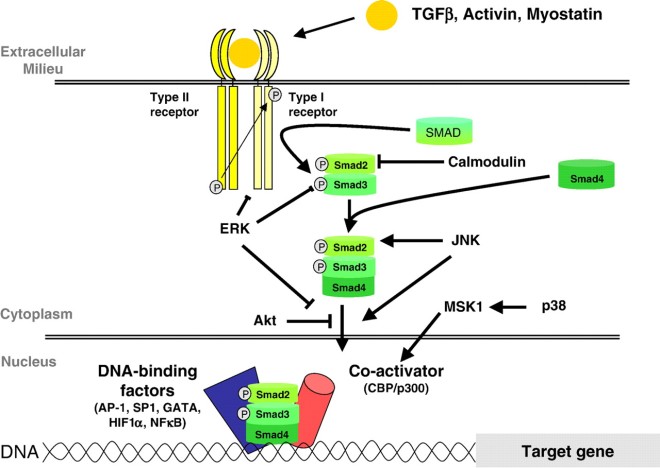
It has been known for some time that scar formation in the heart is largely a consequence of the activation of the TGF-beta signaling pathway (see NG Frangogiannis, Circulation Research 110: 159-173). Inhibition of this pathway can prevent the scar-making cells (fibroblasts) from migrating to the site of damage, dividing, and depositing the protein collagen, which is the main component of heart scars.
An abundant literature on heart scars show that the heart scar plays an important short-term role, but that in the long-run, it prevents the heart from resuming full function because it does not communicate with the rest of the heart muscle cells and does not contract. Therefore, helping the heart get through the first month after the heart attack without a scar is a crucial time.
In a recent paper published by Marbán and his team, cardiospheres were tested in culture and in the heart of mice that had suffered a heart attack. Marbán thought that since the cardiospheres were attenuating scar formation, they must be inhibiting TGF-beta signaling. TGF-beta proteins are secreted by cells and they bind to a receptor complex that then activate intracellular proteins called “SMADs.” These activated SMAD proteins enter the nucleus and activate the transcription of target genes.
In his co-culture experiments, Marbán and others used normal human fibroblasts from the lower layers of human skin and cultured them with and without cardiospheres. The co-culturing experiments showed that without cardiospheres, the dermal fibroblasts made lots of collagen and activated their internal SMAD proteins. When these human dermal fibroblasts were incubated with cardiospheres, their SMAD proteins were largely inactivated and they made very little collagen.
Such a result is not surprising, but how are the cardiospheres doing this? As it turns out, there is an inhibitor of the TGF-beta receptor complex called “endoglin” that can also be secreted known as sE. When Marbán and others examined their cardiospheres, they were secreting a fair amount of sE.
Thus, the production of sE could definitely prevent dermal fibroblasts from activating their SMADs and making collagen, but what if these sE molecules were inactivated? Marbán and others made antibodies against sE and then used them to inactivate the sE made in culture by the cardiospheres. Under such conditions, the cardiospheres no longer were able to prevent SMAD activation in dermal fibroblasts and the fibroblasts made lots of collagen, even in the presence of cardiospheres.
This is all fine and good, but it is in cell culture, and cell culture experiments must always be confirmed by experiments in a living creature. Therefore, Marbán and his colleagues used cardiospheres to treat mice that had suffered a heart attack. As observed before, the cardiosphere-treated mice showed increases in ejection fraction and fractional shortening, and decreases in end-diastolic and end-systolic volume. The cardiosphere-treated animals also had much less scar tissue after one month and greater blood vessel density. Furthermore, the cardiosphere-treated mice did not show the maladaptive enlargement of the heart muscle cells seen in post-heart attack patients. When the heart tissue was assayed one month after treatment, it was clear that the cardiosphere-treated heart tissue showed increased sE expression and much less TGF-beta signaling. The downstream targets of SMAD activation were much less, and SMADs also showed less activation. Expression of the TGF-beta receptors was also decreased.
This paper shows that endoglin expression plays a key role in preserving and healing the heart after a heart attack. Would it be possible to give soluble endoglin to heart attack patients? This remains to be seen.
One caveat with this paper is that human dermal fibroblasts are similar but different from heart fibroblasts. While it is reasonable to suppose that these two cell types react in a similar way to cardiospheres, such a supposition must be rigorously confirmed experimentally.