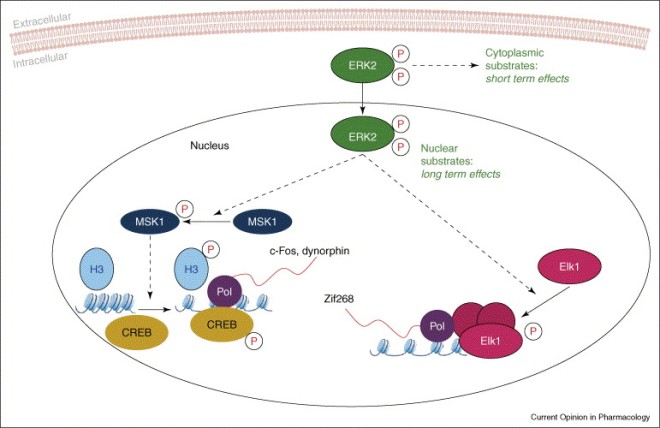
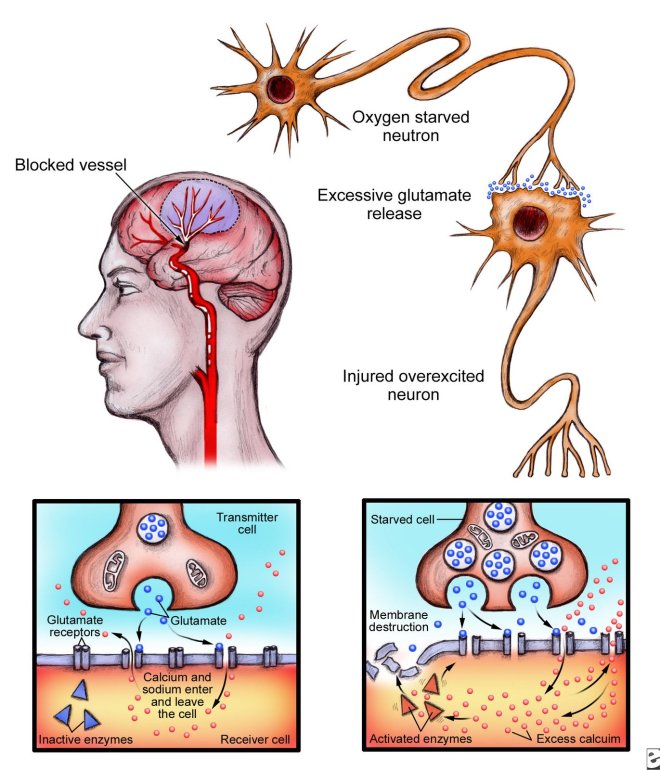
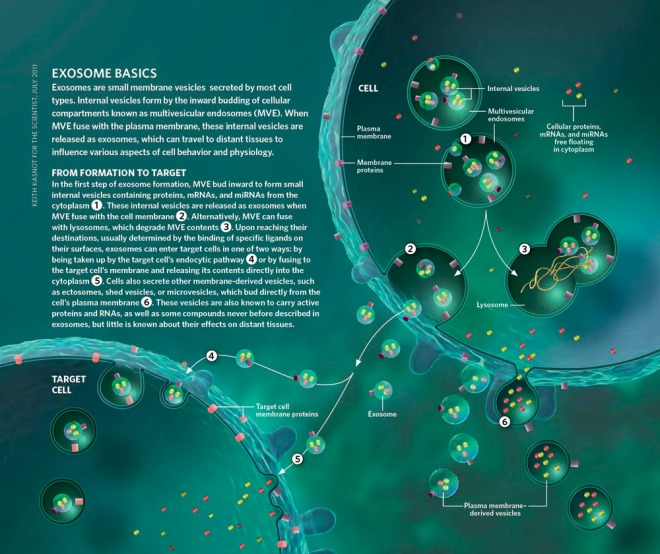
Nature News has an article about VSELs or very small embryonic like stem cells. These cells are supposed to exist in bone marrow and are thought to be extremely rare. However, do they actually exist?
VSEL proponents have extracted them from bone marrow, done experiments with them, and published papers about them. In fact a clinical trial using VSELs has begun in Poland, and the cell-therapy company Neostem in New York is planning another VSEL-based clinical trial in Michigan.
Unfortunately, there is evidence mounting that VSELs do not actually exist. In a paper published on 24 July in Stem Cell Reports, Irving Weissman and his co-workers from Stanford University suggests that the diminutive stem cells are not real. Weissman’s paper is definitely the most thorough paper to date on this issue.
“Weissman’s evidence is a clincher — it is the end of the road for VSELs,” believes Rüdiger Alt, head of research at Vita 34, a private bank for umbilical cord blood in Leipzig, Germany, who last year published the first failure to replicate claims for the cells (see Danova-Alt, R., Heider, A., Egger, D., Cross, M. & Alt, R. PLoS ONE 7, e34899 (2012)).
Robin Smith, who serves as the chief executive at Neostem, disagrees with the conclusions of these papers. She compares the attacks on VSELs to those suffered by Charles Darwin and Nicolaus Copernicus when they proposed their world-changing scientific theories. You must admit that this is more than a little over the top.
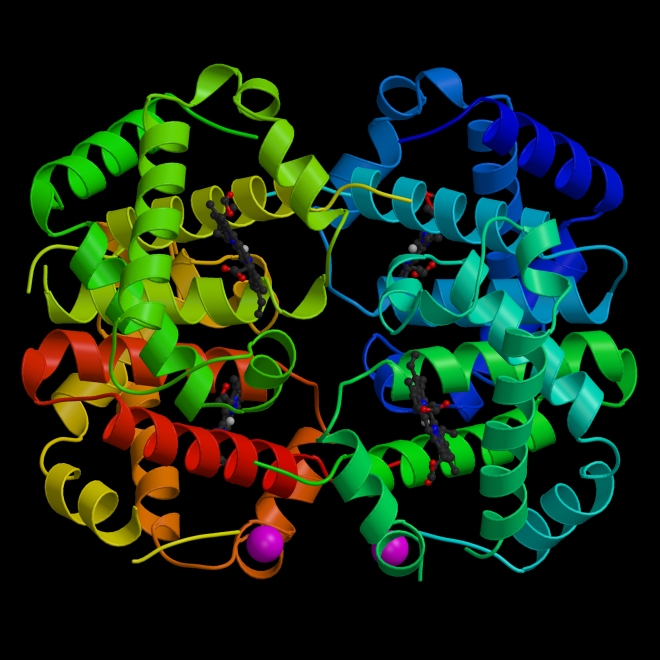
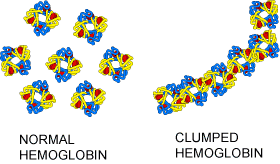
The VSEL battle has been waged for a few years now. VSELs were first described in mouse bone marrow in 2006, by a team led by Mariusz Ratajczak at the University of Louisville in Kentucky (see Kucia, M. et al. Leukemia 20, 857–869 (2006)). Ratajczak and his group and a few others have since generated a literature that characterizes VSELs as rare components of bone marrow and other tissues. According to Ratajczak, VSELs are less than 6 micrometers in diameter and have the ability to differentiate into a diverse range of cell types, including blood, bone, muscle and nerve.
Ratajczak was given a joint position at the Pomeranian Medical University in Szczecin, Poland, in 2006. He also obtained a 10.6 Euro grant (US$14 million) from sources in the European Union for a VSEL research network that involves five institutions. This network last year registered the first human trial of a VSEL preparation, which aims to treat 60 people who have severe angina. Approximately 25% of the participants have already been injected with the preparation.
This VSEL research network, however, had some cracks form when one of Ratajczak’s collaborators, Józef Dulak at the Jagiellonian University in Krakow, was completely unable to find VSELs in his experiments. He published his findings in May (see Szade, K. et al. PLoS ONE 8, e63329 (2013)), but Ratajczak tried to force Dulak out of the VSEL research consortium. Dulak, like Weissman, did not see any molecular signatures associated with pluripotency in any mouse bone-marrow cells that had a diameter less than 7 micrometers across. Weissman’s more extensive analysis found that small bone-marrow derived cells did not aggregate into spheres in vitro, as pluripotent cells typically do; and could not differentiate into blood cells.
Rüdiger Alt and his research team spent 18 months studying human VSELs at Leipzig University. Alt told Nature that he and his group threw in the towel when chromosome analyses showed that the objects they observed in their experiments were not proper cells. “If they had existed, Vita 34 would have been interested to try to commercialize them,” he says. “We wasted a lot of time.” A fourth study, which sought VSELs in blood from umbilical cords, could not show that they were pluripotent either (see Alvarez-Gonzalez, C. et al. PLoS ONE 8, e67968 (2013)).
Together with the University of Louisville, Ratajczak patented his VSEL discovery. In 2007, Neostem acquired exclusive licensing rights and created its Stem For Life Foundation, which promotes adult stem cells as an ethical alternative to embryonic stem-cell therapy. Two years ago, the Vatican, which supports this approach, donated US$1 million to the foundation. This represents the first time the Vatican has entered a joint venture with a trading company. Since that time, the Vatican has hosted two international conferences on adult stem cells, co-organized by Stem For Life and Neostem.
Weissman presented some of his now-published results at an unrelated meeting of the Pontifical Academy of Sciences in Vatican City in April 2012. But he says he is annoyed that representatives of the Catholic Church “act as if they don’t know the scientific data” and have continued to back VSELs.
Russell Taichman, a dental medicine researcher at the University of Michigan in Ann Arbor, said, “I don’t see the controversy — we have seen bone grow” from VSELs in mice. Taichman will be running the Neostem-backed VSEL trial, which was announced in April and is awaiting approval by the US Food and Drug Administration, will look for bone regrowth in dental patients.
The leader of the Polish VESL trial is also unfazed by Weissman’s paper. “Other investigators are just not managing to catch the right cells,” says Wojciech Wojakowski, a cardiologist at the Medical University of Silesia in Katowice.
Diane Krause, who has published evidence that VSELs from mouse bone marrow can differentiate into lung epithelial cells (see Kassmer, S. H. et al. Stem Cells http://dx.doi.org/10.1002/stem.1413 (2013)), agrees. “I can only say that we manage to see these cells,” she says. “One of our postdocs went to the Ratajczak lab and learnt the technique properly.”
Ratajczak thinks that all the published criticisms come down to investigators lacking the technical skill to harvest the correct cells. “Weissman has never visited my lab to witness exactly how we carry out the method,” he says. Weissman counters that “science has to be reproducible, and methods described so that others can reproduce them”.
Neostem has started to moderate its VSEL statements. In an e-mail, Smith told Nature that the company “has studies in progress to determine, with robust data, whether or not VSELs have characteristics of pluripotent cells.”
The debate exemplifies problems often seen in adult stem-cell research, in which a trial can end up being based on inadequate data, says Rudolf Jaenisch, a stem-cell researcher at the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts. “It’s even worse when it gets the blessing of the church.” In all honesty, who listens to the Catholic Church anymore in the scientific community? That was a cheap shot. The Church should stop supporting VSEL research if it is clear that they do not exist, but it is possible that Weissman and his colleagues are wrong and Ratajczak and his colleagues are correct (not likely, but possible). Therefore, leave the Church out of this.
I have summarized some data from papers reporting on VSELs on this blog. Unfortunately, there are only a few papers on VSELs, and the number of people who doubt their existence is growing. It is certainly possible that the VSEL research group have a technique for isolating them that is difficult to replicate. I am reminded of the Multipotent Adult Progenitor Cells that were first isolated and characterized by Catherine Verfaillie. Her MAPC reports were met with some skepticism when other labs could not routinely reproduce them. However, despite mislabelings that occurred in her original paper, she has been somewhat vindicated (see J Clin Invest. 2002 May;109(10):1291-302). Thus it is possible that these other labs simply cannot replicate the isolation procedure properly.
However, Weissman is right when he says that science must be reproducible. I think Dr. Weissman should send one of his postdocs or graduate students to Ratajczak’s lab to learn their techniques and then try to reproduce them. If that fails, then it seems rather unlikely that VSELs exist. However, if other labs continue to find VSELs and do experiments with them, and if they prove to be efficacious in clinical trials, then it seems to me that VSELs do exist, but they are simply quite rare in bone marrow and difficult to isolate.
Also, I think Weissman’s shot at the Catholic Church was gratuitous and mean-spirited. The church officials have sunk a lot of church money sink into VSELs. Of course they want them to exist. It is entirely possible that Weissman and his colleagues are incorrect. He is welcome to disagree with them, but he should also show some charity to the Vatican folk, who graciously hosted him at their conference.
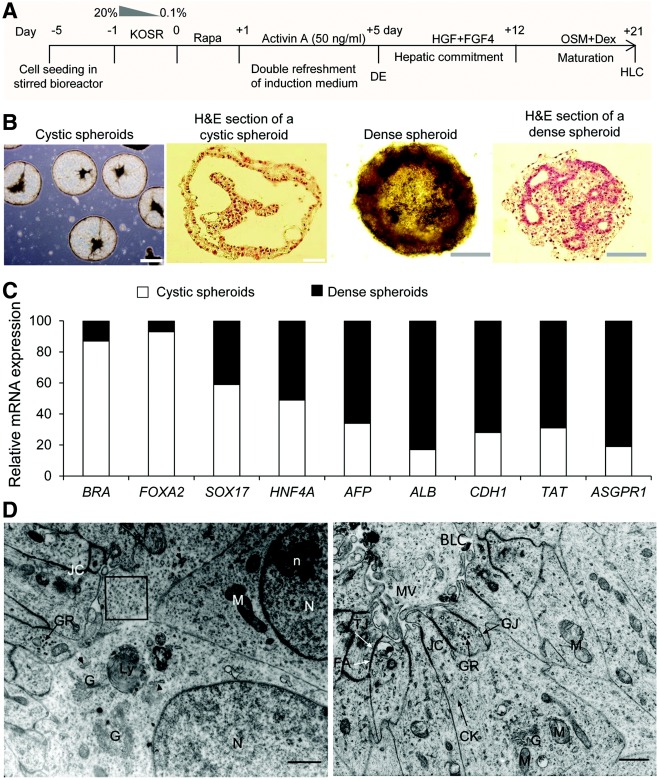
![Induction of hiPSCs into definitive endoderm. (A) Diagrammatic representation of the experimental groups for endoderm induction of hiPSCs in the bacterial dish static suspension, which include rapamycin (Rapa) “priming” and activin A “inducing” phases, and positive control groups of hiPSCs cultured in the absence of Rapa in suspension or adherent cultures in the presence of activin A. (B) Gene expression analysis of hiPSCs induced into endodermal cells. Quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) showed no significant differences in SOX17 and FOXA2 [definitive endoderm (DE) markers], and BRA (mesoendoderm marker) in all groups. SOX7 (visceral endoderm marker) was expressed at a low level. Influence of the refreshment strategy of the induction medium on DE formation in suspension cultures by qRT-PCR (C), immunostaining (D), and flow cytometry (E). We compared single (SR), double (DR), and triple (TR) refreshment of induction medium per 24 h for 4 days after Rapa administration in the static suspension of hiPSCs. Scale bar: 100 μm. The target gene expression level in qRT-PCR was normalized to GAPDH and calibrated with (presented relative to) hiPSCs. Data are presented as mean±SD. Statistical analysis as determined by one-way ANOVA with Tukey's post hoc test, n=3 for B, C, and E. *P<0.05, **P<0.01.](https://beyondthedish.files.wordpress.com/2013/07/definitive-endoderm-from-ipscs.jpeg?w=660)