Wesley Smith at National Review Online has been keeping tabs on the reporting of the Cell paper by Shoukhrat Miltalipov from the Oregon Health and Science University. The misrepresentation has been extensive but it’s not really all that surprising given the ignorance and lack of clear thinking on this issue. Nevertheless, Smith has kept up his yeoman’s work, cataloging the factual errors for reporters in multiple publications.
For his first example, see here, where Loren Grush on Fox News.com wrote:
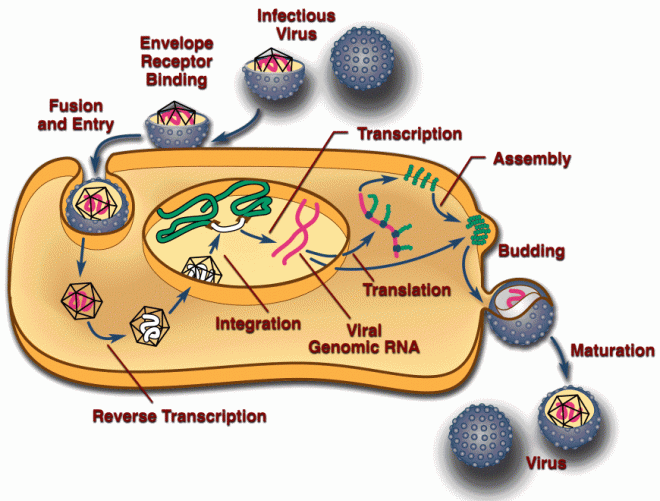
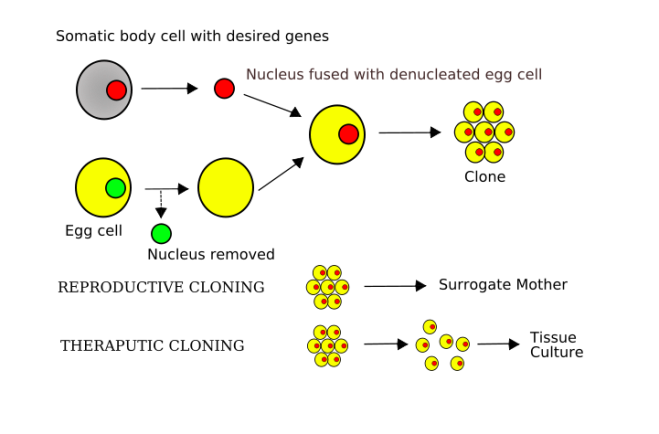
Through a common laboratory method known as somatic cell nuclear transfer (SCNT), ONPRC scientists, along with researchers at Oregon Health & Science University, essentially swapped the genetic codes of an unfertilized egg and a human skin cell to create their new embryonic stem cells…The combination of the egg’s cytoplasm and the skin cell’s nucleus eventually grows and develops into the embryonic stem cell.
Grush, as Smith points out, is quite wrong. Introducing a nucleus from a body cell into the unfertilized egg and inducing it does not turn the egg into embryonic stem cells, but turns it into a zygote. The zygote them undergoes cleavage (cell division) until it reaches the early/mid blastocyst stage 5-6 days later, then immunosurgery is used to isolated the inner cell mass cells, after which they are cultured. Somatic cell nuclear transfer is a stand-in for fertilization. It produces an embryo and all the redefinition in the world will not change that.
Next comes my favorite newspaper, the Wall Street Journal, which normally has decent to pretty good scientific reporting, but this one story from Gautam Naik contains a real howler:
Scientists have used cloning technology to transform human skin cells into embryonic stem cells, an experiment that may revive the controversy over human cloning. The researchers stopped well short of creating a human clone. But they showed, for the first time, that it is possible to create cloned embryonic stem cells that are genetically identical to the person from whom they are derived.
As Smith points out, Miltalipov and others did not stop short of creating a human clone, then explicitly made a cloned human embryo and therefore made a cloned young human being.
Then there is this humdinger from an online Australian news report:
US researchers have reported a breakthrough in stem cell research, describing how they have turned human skin cells into embryonic stem cells for the first time. The method described on Wednesday by Oregon State University scientists in the journal Cell, would not likely be able to create human clones, said Shoukhrat Mitalipov, senior scientist at the Oregon National Primate Research Center. But it is an important step in research because it doesn’t require the use of embryos in creating the type of stem cell capable of transforming into any other type of cell in the body.
Oh my gosh, folks the paper describes the production of cloned embryos expressly for the purpose of dismembering them and destroying them. This “doesn’t require the use of embryos” crap reveals a very basic ignorance of how the experiment was done. See Smith’s excellent post for more details.
Then there is this story from one of my least favorite papers, the LA Times:
Some critics continue to argue that it’s unethical to manipulate the genetic makeup of human eggs even if they’re unfertilized, and others warn about potential harm to egg donors. The biggest ethical issue for the OHSU team, though, is that it artificially created a human embryo, albeit one that was missing the components needed for implantation and development as a fetus.
Come on people! The cloned embryo does not have the components needed to implant because there is no womb into which it can be implanted. Dolly was made the same way. Surely Dolly had the components required to implant. The problem here is one of will, since these embryos were made to be destroyed. Not capacity. What was done to those embryos was dismemberment. Would we object if they were toddlers?
Just to show that obfuscation is not wholly an American news feature, there is this story from the German newspaper Deutche Welle:
Scientists, for the first time, have cloned embryonic stem cells using reprogrammed adult skin cells, without using human embryos…The process used by Mitalipov is an important step in research because it does not require killing a human embryo–that is, a potential human being–to create transformative stem cells.
As Smith points out, this research made a human embryo that was then killed to make embryonic stem cells. Calling this research humane is to redefine humane to the point of absurdity.
Finally this jewel of blithering ignorance from bioethicist Jonathan Moreno in his column in the Huffington Post:
Despite some confused media reports, the Oregon scientists did not clone a human embryo but a blastocyst that lacks some of the cells needed to implant in a uterus.
And you wonder why people like me have lost all faith in American bioethics. As a developmental biologist, this one just grates on me. A blastocyst has two cell populations; an outer trophectoderm composed of trophoblast cells that will form the placenta and the inner cell mass cells on the inside of the embryo, which will form the embryo proper and a few placental structures. To be a blastocyst is to have the equipment to implant.
To drive the nail into the coffin, Smith quotes the father of embryonic stem cells James Thomson from an MSNBC interview:
See, you are trying to redefine it away…If you create an embryo by nuclear transfer, if you gave it to somebody who didn’t know where it came from, there would be no test you could do on that embryo to say where it came from. It is what it is. By any reasonable definition, at least as some frequency, you are creating an embryo. If you try to redefine it away, you are being disingenuous.
Check out Smith’s posts. They are all worth reading. Maybe the press will learn some embryology, but I doubt it.
Postscript: Brendan P. Foht writes at the Corner on National Review Online that in 2010 Shoukhrat Mitalipov, the leader of the Oregon cloning team, reported that he had achieved a single pregnancy using cloned monkey embryos that were made with exactly the same technology as was employed with human eggs in his 2013 Cell paper. The fetus developed long enough to have a heartbeat detectable through ultrasound. Although the pregnancy failed after 81 days (about half the normal gestation period for that species), the fact that a pregnancy would develop so far indicates that reproductive cloning of primates is in principle possible. This definitively shows that all this talk about the embryos made in Mitalipov’s lab not being able to implant is pure drek.