Michael Rudnicki, who has done pioneering work in muscle stem cell biology and muscle regeneration, and whose work has been featured several times on this blog, has struck again. Rudnicki, who serves as director of the Regenerative Medicine Program at The Ottawa Hospital and a professor at the University of Ottawa and holds the prestigious Canada Research Chair in Molecular Genetics, teamed up with workers from the Sprott Centre for Stem Cell Research and the Sinclair Centre for Regenerative Medicine to investigate the role of muscle-specific stem cells in patients who suffer from Duchenne muscular dystrophy. This new earth-shaking study, which was published in the journal Nature Medicine (November 16, 2015), has changed the way we think about muscular dystrophy and will almost certainly force people to rethink the treatments and cures for this dreadful disease.
According to this new study, Duchenne muscular dystrophy directly affects muscle stem cells, and is, largely a disease of muscle stem cells.
Rudicki said: “For nearly 20 years, we’ve thought that the muscle weakness observed in patients with Duchenne muscular dystrophy is primarily due to problems in their muscle fibers, but our research shows that it is also due to intrinsic defects in the function of their muscle stem cells. This completely changes our understanding of Duchenne muscular dystrophy and could eventually lead to far more effective treatments.”
Muscular dystrophy comes in several different forms, but the predominant sign of muscular dystrophy is progressive muscle weakness. Altogether, muscular dystrophy refers to a group of more than 30 genetic diseases, all of which cause progressive weakness and degeneration of skeletal muscles used during voluntary movement. Approximately half of all who suffer from muscular dystrophy have Duchenne muscular dystrophy (DMD). Because muscular dystrophy results from mutations in the dystrophin gene, which is on the X chromosome, the vast majority of muscular dystrophy patients are male. Girls can be carriers of muscular dystrophy and can be mildly affected.
Interestingly, somewhere around one-third of boys who suffer from DMD have no family history of the disease. Because the dystrophin gene is so large, spontaneous mutations in it are probably relatively common.
The signs and symptoms typically appear between the ages of 2 and 3, and may include frequent falls, difficulty getting up from a lying or sitting position, trouble running and jumping, a strange, shuffling way of walking or having a tendency to walk on their toes, calf muscles that are abnormally large, muscle pain and stiffness, and some learning disabilities.
Becker muscular dystrophy (BMD) has signs and symptoms that are largely similar to those of DMD, but BMD tends to be a milder form of the disease that progresses more slowly. Symptoms typically begin in the teens but, some patients may not experience symptoms until their mid-20s and some may not experience symptoms until later.
There are also several different types of muscular dystrophy-type diseases. Steinert’s disease or myotonic muscular dystrophy, which is characterized by an inability to relax muscles at after contractions, is the most common form of adult-onset muscular dystrophy. The first muscles to be affected are the muscles of the face and neck. Facioscapulohumeral muscular dystrophy affects the muscles of the face and shoulders, where symptoms first begin. When patients with facioscapulohumeral raise their arms, their shoulder blades noticeably protrude. This disease may first manifest itself in children, teenagers as late as age 40. This disease tends to affect one side more than the other.
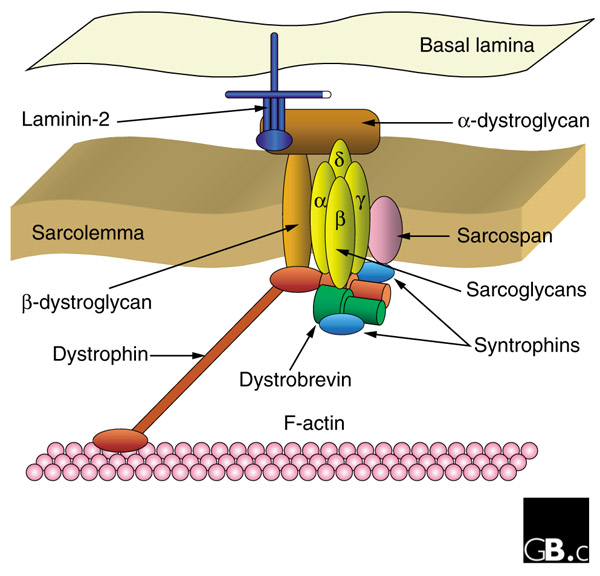
Limb-girdle muscular dystrophy affects the muscles of the shoulders and hips. There are over 20 inherited forms of this disease, and because this condition is not due to mutations in dystrophin, but to mutations in genes that encode proteins that interact with dystrophin, the inheritance of limb-girdle muscular dystrophy is not sex-linked. Some forms of this disease are recessive and some are dominant. Patients with this type of muscular dystrophy usually trip more often because they have trouble raising the front part of their feet. Some autosomal recessive forms of the disorder are now known to be due to a deficits in proteins called sarcoglycans or dystroglycan.
Congenital muscular dystrophy is extremely varible and is probably a cluster of several different diseases caused by mutations in different genes. Some of types of congenital muscular dystrophy show sex-linked inheritance while others do not. Most cases of congenital muscular dystrophy result from the absence of a muscle protein called merosin, which is found in the connective tissue that surrounds muscle fibers. Other types of congenital muscular dystrophy have normal merosin and still others result from abnormal motor neuron migration. Clinically, this disease is also extremely variable and can manifest itself at birth or before age 2, progress slowly or rapidly, and cause mild disability or severe impairment.
Muscular dystrophy affects all ethnic groups and occurs globally. It affects around 1 in every 3,500 to 6,000 male births each year in the United States. DMD affects approximately one in 3,600 boys.
Because DMD results from mutations in the dystrophin gene, the vast majority of muscular dystrophy research was based on a simple model in which the Dystrophin protein played a structural role in the structural integrity of muscle fibers. Abnormal versions of the Dystrophin protein caused the muscle fibers to become damaged and die as a result of contraction. Dystrophin anchors the cytoskeleton of the muscle fibers, which are essential for muscle contraction, to the muscle cell membrane, and then to the extracellular matrix outside the cell that serves as a foundation upon which the muscle cells are built.
However in this current study, Rudnicki and his team discovered that muscle stem cells also express the dystrophin protein. This is a revelation because Dystrophin was thought to be protein that ONLY appeared in mature muscle. However, in this study, it became exceedingly clear that in the absence of Dystrophin, muscle stem cells generated ten-fold fewer muscle precursor cells, and, consequently, far fewer functional muscle fibers. Dystrophin is also a component of a signal transduction pathway that allows muscle stem cells to properly ascertain if they need to replace dead or dying muscle. Muscle stem cells repair the muscle in response to injury or exercise by dividing to generate precursor cells that differentiate into muscle fibers.
“Muscle stem cells that lack dystrophin cannot tell which way is up and which way is down,” said Dr. Rudnicki. “This is crucial because muscle stem cells need to sense their environment to decide whether to produce more stem cells or to form new muscle fibres. Without this information, muscle stem cells cannot divide properly and cannot properly repair damaged muscle.”
Even though Rudnicki used mice as a model system in these experiments, the Dystrophin protein is highly conserved in most vertebrate animals. Therefore, it is highly likely that these results will also apply to human muscle stem cells.
Treatment for DMD patients is limited to steroids to decrease muscle inflammation and muscle cell death, and physical therapy to increase muscle use and prevent muscle atrophy. These approaches only delay the progression of the disease and alleviate symptoms. Gene therapy experiments and trials are in progress and even show some promise, but Rudnicki’s work tell us that gene therapy approaches must target muscle stem cells as well as muscle fibers if they are to work properly.
“We’re already looking at approaches to correct this problem in muscle stem cells,” said Dr. Rudnicki. “I’m not sure if we will ever cure Duchenne muscular dystrophy, but I’m very hopeful that someday in the future, we will have new therapies that correct the ability of muscle stem cells to repair the muscles of afflicted patients and turn this devastating, lethal disease into a chronic but manageable condition.”
This paper has received high praise from the likes of Ronald Worton, who was one of the co-discovers of the dystrophin gene with Louis Kunkel in 1987. Worton later served as Vice-President of Research at The Ottawa Hospital from 1996 to 2007.
“When we discovered the gene for Duchenne muscular dystrophy, there was great hope that we would be able to develop a new treatment fairly quickly,” said Dr. Worton, who is now retired. “This has been much more difficult than we initially thought, but Dr. Rudnicki’s research is a major breakthrough that should renew hope for researchers, patients and families.”