I wrote this review article for the Mesenchymal Stem Cell site. Unfortunately, this site has now become defunct. Therefore, I have moved it here for your enjoyment:
“Critical Distinctions between Mesenchymal Stem Cells from Bone Marrow and Alternative Sources”
Michael Buratovich Ph.D (Author)
Supplied Courtesy of BioInformant Worldwide, LLC
http://www.BioInformant.com
Introduction
Mesenchymal stem cells (MSCs) are adult, multipotent stem cells that have been isolated from circulating blood (Kuznetsov et al 2001), umbilical cord blood (Beibacket al 2004; Lee et al 2004b), placenta (Iguraet al 2004), heart (Warejckaet al 1996), amniotic fluid (Tsai et al2004), adipose tissue (Katzet al 2005), synovium (Fickert et al 2003), skeletal muscle (Younget al 1995), pancreas (Hu et al 2003), deciduous teeth (Estrelaet al 2011), and bone marrow (Charbord 2010). Bone marrow-derived MSCs (BMSCs) are the most heavily-studied of all MSCs, and, therefore, tend to be the standard against which MSCs from other sources are evaluated. BMSCs can differentiate into osteoblasts, chondrocytes, adipocytes, fibroblasts, hepatocytes, neural cells, etc., and can give rise to cartilage (Kadiyala et al 1997), bone (Bruder et al 1997; 1998), tendon (Young et al 1998), muscle (Galmiche et al 1993; Ferrari et al 1998), and many other tissues. Do MSCs from tissues other than bone marrow have similar differentiation potentials, and if not how does the potency of these MSCs from alternative sources compare with those from bone marrow? Fortunately stem-cell scientists have examined this question in some detail, but a central question remains: Do MSCs from diverse bodily locations represent distinct or the same cell types?
If MSCs throughout the body are similar cell types then we would expect them to have similar embryological origins. However, this is not the case, since MSCs develop from several different embryonic tissues. The first wave of MSCs arises from Sox-1-expressing neuroepithelial cells during embryonic development. However, later MSCs come from multiple sources (Takashima et al 2007), including neural crest cells (Nagoshi et al 2008; Morikawaet al 2009). Therefore, MSCs from various tissues almost certainly have distinct embryological origins. Additionally, MSCs are located in different sites in the body, and are influenced by specific microenvironments. Thus MSCs from different tissue sources might represent distinct cell types, and could potentially display distinct differentiation profiles and express particular genes. Despite these differences in developmental origin and environmental influences, MSCs from various sources have very similar morphologies and share a common array of surface markers (Mitchell et al 2003; Lee et al 2004a; Wang et al 2004; Tsai et al 2007). However, several studies have established that MSC populations are rather heterogeneous (Dominici et al 2009), and, therefore, surface markers expressed on some cells of an MSC population are not always expressed in all the cells of that population (Mafi et al 2011). Also, the growth kinetics of cultured MSCs differs remarkably with respect to their source (Kang et al 2004b; Yoshimura et al 2007; Troyer and Weiss 2008).
Despite the shared array of cell surface markers, presently there are no cellular markers or cell surface proteins that are unique to MSCs. In order to provide a more unified approach to MSC biology, the International Society of Cryotherapy has proposed three criteria for the identification of MSCs. Under these criteria, MSCs must: (1) be plastic-adherent when maintained in standard culture conditions; (2) express the following cell surface molecules CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface molecules, and; (3) be able to differentiate into osteoblasts, adipocytes and chondroblasts in vitro (Dominici et al 2006). Despite these definitions, flow cytometric analyses of MSCs from several different populations have shown some significant differences in cell surface markers (Boeuf and Richter 2010). For example, even though the absence of CD34 is generally considered a criterion for the definition of MSCs, various investigators have reported low expression of CD34 in ADSCs (ADSCs; De Ugarte et al 2003a; Rebelatto et al 2008; Roche et al 2009) and BMSCs (Zvaifler et al 2000; Gronthos et al 2003; Yu et al 2010). Likewise, many investigators have shown that MSCs from multiple sources do not express CD45 (Zvaifler et al 2000; Zuk et al 2002; Igura et al 2004; Dominici et al 2006; Wongchuensoontorn et al 2009), but BMSCs are CD45 positive (Yu et al 2010).
Other cell marker differences include CD271,which shows high levels of expression in BMSCs and ADSCs (Jones et al 2002; Quirici et al 2010), but is not expressed in synovial membrane MSCs (SMSCs; De Bari et al 2001; Van Landuyt et al 2010). Another molecule that is highly expressed in the vast majority of MSC population is STRO-1 (Gronthos et al 1991; Simmons and Torok-Storb 1991; Gronthos et al 1994; Gronthos et al 1999; Stewart et al 1999; Walsh et al 2000; Zuk et al 2002; Miura et al 2003; Kadar et al 2009), but other studies have shown that ADSCs are STRO-1 negative (Gronthos et al 2001). Signal transduction receptors also show varied expression in distinct MSC populations. For example, platelet-derived growth factor receptor (CD140a/PDGFRα) is involved in proliferation and migration of osteoblasts and MSCs. This receptor is much more highly expressed in SMSCs than BMSCs (Nimura et al 2008). Finally the vascular cell adhesion molecule CD106/VCAM1, which is involved in hematopoietic stem cell homing (Simmons et al 1992), is more highly expressed in BMSCs than ADSCs (De Ugarte et al 2003a; Kern et al 2006; Rider et al 2008; Roche et al 2009). This cell surface difference almost certainly is related to the specific microenvironment in which BMSCs are found and their specific roles in maintaining hematopoietic stem cell growth.
Comparative gene array analyses of MSCs from different sources have revealed some differences in gene expression between these distinct MSC populations, but overall the gene expression profiles between these cells are relatively similar (Winter et al 2003; Lee et al 2004a; Djouad et al 2005; Wagner et al 2005; Aranda et al 2009; Jansen et al 2010). Proteomic comparisons of distinct MSC populations using two-dimensional gel electrophoresis analysis came to very similar conclusions (Roche et al 2009). MSCs from intra-articular tissues (synovial membrane and anterior cruciate ligament) and chondrocytes show gene expression profiles that were more similar to each other than to MSCs from extra-articular locations (Segawa et al 2009). These data suggest that MSCs from varied sources probably represent similar, but distinct cell types that express a core of common genes, but also clusters of distinct genes. These gene expression differences convey different differentiation potentials upon specific MSC populations and varied requirements for these particular MSC populations to differentiate into specific cell types (Gimble et al 2008; Rastegar et al 2010).
MSC Differentiation
With respect to the differentiation potential of MSC populations, the general rule of thumb is the closer the MSC source tissue is to the target tissue, the more effectively that particular MSC population differentiates into the target tissue. A few examples should suffice. Yoshimura and colleagues found that rat SMSCs derived from the synovial tissue of the knee, which is closest to the target tissue of chondral cartilage, formed cartilage better than BMSCs, ADSCs, or MSCs from periosteum or muscle (Yoshimura et al 2007). Likewise, gene expression profiles of human BMSCs or umbilical cord-derived MSCs (UCSCs from Wharton’s jelly) definitively showed that BMSCs express a variety of osteogenic genes (RUNX2, DLX5 and NPR3) not observed in UCSCs. Under osteogenic induction, BMSCs produced far more bone than UCSCs. However, UCSCs express angiogenesis genesand fewer genes involved in the immune response than BMSCs, suggesting that UCSCs are superior for allogeneic transplantation. When cocultured with allogeneic macrophages,UCSCs prevented the macrophages from producing immunomodulatory cytokines tumor necrosis factor and Interleukin-6 (Hsieh et al 2010). Finally, Niemeyer and coworkers showed that BMSCs and ADSCs formed bone with similar efficiencies in vivo (Niemeyer et al 2007), but in animals studies, BMSCs produced better repair of tibial osteochondral defects in sheep when compared to ADSCs (Niemeyeret al 2010).
MSC Chondrogenesis
Initiation of cartilage development during animal development begins with the condensation of mesenchymal precursor cells (Woods, Wang and Beier 2007). These cell-cell contacts are mediated by N-cadherin, whose expression is highly upregulated in human MSCs after being subjected to chondrogenic induction (Tuli et al 2003). N-cadherin is required for chondrogenesis of chick limb mesenchymal cells in vitro and in vivo (Oberlender and Tuan 1994). Prior to MSC condensation prechondrocytic MSCs secrete extracellular matrix rich in hyaluronic acid, collagen type I and IIa. Initiation of MSC condensation also correlates with the expression of neural cadherin (N-cadherin) and neural cell adhesion molecule (N-CAM). The secreted signaling molecule transforming growth factor-β (TGF-β) is one of the earliest signals in chondrogenic condensation. TGF-β activates production of the extracellular matrix protein fibronectin, which up-regulates N-CAM, and also stimulates the synthesis of Sox transcription factors (Sox-5, -6 and -9), which are essential for cartilage formation. Other extracellular matrix molecules made by chondrogenic MSCs include tenascins, thrombospondins, and cartilage oligomeric protein (COMP). These extracellular matrix molecules interact with cell adhesion molecules to activate intracellular signaling pathways that initiate the transition from chondroprogenitor cells to fully committed chondrocytes. Proliferating chondroprogenitor cells synthesize hyaluronan, collagen II, IX and XI, and the cartilage-specific proteoglycan core protein (or chondroitin sulfate proteoglycan 1) known as aggrecan. Aggrecan (encoded by the ACANgene) is a member of the aggrecan/versican proteoglycan family, and is the most predominant proteoglycan in the extracellular matrix of articular cartilage. Aggrecan helps cartilage withstand compression. N-cadherin and N-CAM expression fade and disappear in differentiating chondrocytes (Golding, Tsuchimochi and Ijiri 2006).
When grown under chondrogenic conditions, MSCs in monolayer culture respond by condensing into high-density three-dimensional cell aggregates (Winter et al 2003). In order to realistically recapitulate chondrogenesis in culture, researchers deposit centrifuged MSC pellets that contain ~200,000 – 500,000 cells in a two-dimensional culture. This culture system, which is one of the most widely used in chondrogenesis research, is called a pellet, aggregate or spheroid culture. To induce chondrogenesis, pellets are cultured in a basal medium (typically low- or high-glucose Dulbecco’s Modified Eagles Medium, otherwise known as DMEM, or fetal calf serum) that contains dexamethasone, ascorbate, proline, insulin, transferrin and selenous acid (Johnstone et al 1998; MacKay et al 1998; Puetzer, Petitte and Loboa 2010). Classically, the growth factor used to induce chondrogenesis in this type of medium is 10 ng/ml of transforming growth factor-β (TGF-β). TGF-β1, 2, and 3 are the only well-established full inducers of chondrogenesis that, when added as single factors, induce proteoglycan and collagen type II deposition (MacKay et al 1998; Barry et al 2001). Other chondrogenic inducers have been described; bone morphogen protein-2 (BMP-2) for BMSCs (Schmitt et al 2003) and BMP-6 for ADSCs (Estes, Wu and Guilak 2006). However, other studies have failed to confirm the chondrogenic efficacy of these two growth factors (Winter et al 2003; Indrawattana et al 2004; Xu et al 2006; Hennig et al 2007; Weiss et al 2010), and there is even a chance that these two growth factors might only work in a donor-specific fashion.BMP-2, -4, and -6, and insulin-like growth factor-1 (IGF-1) seem to promote chondrogenesis in MSCs when given in combination with TGF-β (Schmitt et al 2003; Im, Shin and Lee 2005; Sekiya et al 2005; Liu et al 2007).
Presently, a significant controversy exists over whether ADSCs or BMSCs are better sources for orthopedic tissue repair (Frisbee et al 2009). Both BMSCs and ADSCs have been successfully differentiated into chondrocytes in vitro (John stone et al 1998; Erickson et al 2002) and used for cartilage repair in vivo (Wakitani et al 1994; Im et al 2001; Centeno et al 2011). However, harvesting adipose tissue is much less painful than bone marrow aspirations, which makes ADSCs much more preferable for orthopedic therapies.
With respect to MSC chondrogenesis (cartilage induction), several studies have reported relatively robust chondrogenesis by ADSCs in two-dimensional (Zuk et al 2002; Erickson et al 2002; Gimble and Guilak 2003) and three-dimensional culture systems (Awad et al 2004; Estes, Wu, and Guilak 2006). However, several head-to-head comparisons of BMSCs and ADSCs have produced contradictory results, with some studies reporting equivalent chondrogenic capacities (Zuk et al 2001; De Ugarte et al 2003b; Rebelatto et al 2007), but many others concluding that human and equine BMSCs show superior chondrogenic ability (Winter et al 2003; Im, Shin and Lee 2005; Sakaguchi et al 2005; Vidal et al 2008). Because the same MSC populations from different donors show different differentiation potentials (Bieback et al 2004; Chang et al 2006a Kern et al 2006), head-to-head comparisons of donor-matched MSC populations are essential in order to compare the chondrogenic potential of MSCs that share the same genetic background. Such donor-matched studies have consistently shown that BMSCs show superior chondrogenic potential over ADSCs (Huang et al 2005; Afizah et al 2007). Additionally, gene array studies indicate that during chondrogenic induction, BMSCs show gene expression profiles that more closely resemble native cartilage than ADSCs (Winter et al 2003). If grown in three-dimensional culture, which is thought to be an essential aspect of chondrogenic differentiation (Johnstone et al 1998; Yoo et al 1998; Erickson et al 2002), once again BMSCs outperform ADSCs if seeded in a hyaluronic acid scaffold (Jakobsen et al 2010) or encapsulated in alginate (Mehlhorn et al 2006). BMSCs also show superior chondrogenesis to UCSCs in a three-dimensional culture in which cells were seeded in a polygycolic acid (PGA) matrix (Wang et al 2009).
These data do not necessarily mean that BMSCs are the best cartilage-making MSCs in the body. First of all, head-to-head comparisons treated both MSC populations with the same chondrogenic induction protocol, which implicitly assumes that culture conditions optimized for BMSCs are also be optimal for ADSCs. This assumption, however, ignores the intrinsic differences between these two MSC populations. Kim and Im have shown that ADSCs display a chondrogenic potential equal to that of BMSCs if ADSCs are treated with higher concentrations of growth factors (Kim and Im 2009). Additionally, Diekman and colleagues have shown that chondrogenesis of BMSCs and ADSCs is highly dependent on the presence and concentration of particular growth factors, the presence or absence of serum, and the composition of the scaffold in which the cells are embedded for the chondrogenic induction. ADSCs made significantly more aggrecan in response to BMP-6 than to TGF-β, but the opposite was true for BMSCs. Likewise, ADSCs produced more type II collagen in the presence of serum whereas BMSCs produced more type II collagen without serum. Finally when seeded in alginate beads, the quantity of glycosaminoglycan (GAG) made by BMSCs were significantly higher in the dual-growth factor cocktail of TGF-β and BMP-6 as compared to TGF-β alone. However, when these same cells were grown in a cartilage-derived matrix, those grown in the TGF-β-alone cocktail had higher viability and produced higher amounts of GAG when compared to those grown in dual cocktail (TGF-β + BMP-6). Thus the growth scaffold greatly influences the response of MSCs to particular growth factors, but these data also underscore that BMSCs and ADSCs are probably distinct cell types (Diekman et al 2010).
Secondly, keeping with the original rule that the closer the source tissue is to the desired target tissue, the more effectively MSCs from those tissue sources differentiate into the target tissue, Sakaguchi and colleagues showed that MSCS from bone marrow, synovium, and periosteum made more cartilage than ADSCs or skeletal muscle-derived MSCs, but SMSCs clearly made the most cartilage (Sakaguchi et al 2005). Interestingly, this result was replicated in rat MSCs (Yoshimura et al 2007). Equine BMSCs, however, do show superior chondrogenesis to UCSCs and MSCs from amniotic fluid (Lovati et al 2011), and human fetal and adult BMSCs exceed the chondrogenic potentials of fetal lung-, and placenta-derived MSCs (Bernardo et al 2007).
The varied responses of MSCs from various sources to different growth factors also have been well documented. For example, TGF-β alone is sufficient for chondrogenesis of BMSCs (Afizah et al 2007), but not ADSCs (Awad et al 2003: Estes, Wu and Guilak 2005). Additionally, the combination of TGF-β and dexamethasone stimulates chondrogenesis in BMSCs, but in ADSCs, TGFβ is required for chondrogenesis but dexamethasone tends to suppress chondrogenesis (Awad et al 2003). The reduced chondrogenic induction of ADSCs by TGF-β is probably due to reduced expression of the TGF-β receptor in these cells. However, BMP-6 treatment induces expression of the TGF-β receptor ALK-5 in ADSCs and combined application of TGF-β and BMP-6 restores chondrogenesis in this MSC population (Hennig et al 2007). A published protocol to successfully differentiate ADSCs into chondrocytes makes use of the combination of TGF-β and BMP-6 (Estes et al 2010).
Differential responses to BMP-6 are also observed in different types of MSCs. As previously mentioned, BMP-6 strongly induces chondrogenesis in ADSCs, but not in BMSCs. BMP-6 in combination with TGF-β inhibits hypertrophy in ADSCs (Estes, Wu and Guilak 2003), but in BMSCs, BMP-6 promotes hypertrophy and endochondral ossification (Sekiya, Colter and Prockop 2001; Sekiya et al 2002; Indrawattana et al 2004).
These varied responses to growth factors by distinct MSC populations might also be a reflection of the assorted levels of “stemness” found among the cells of each MSC population. As previously noted, MSC populations tend to be highly heterotropic, and clonal analyses of ADSCs have shown that these cell populations are a mixture of cells that can form bone, cartilage and fat (tripotent), those that can only form two of these tissues (bipotent), and others that can only form only cell type (monopotent). The ratios of these tripotent, bipotent to monopotent clones seems to vary from study to study. Guilak and colleagues found that 21% of ADSCs clones were tripotent and approximately 30% were bipotent (Guilak et al 2006), but Zuk and others found that only 1.4% of all ADSC clones were tripotent (Zuk et al 2002). The disparities between these studies seem to be due to the media conditions used, the age of the adipose tissue donors, and the overall design of the experiment. However, these studies certainly show that distinct MSC populations consist of cells at varying levels of “stemness,” with some being more committed to a particular cell type and others being less developmentally committed to a particular cell fate. The heterogeneity of these populations almost certainly influences the response of these cell populations to particular growth factors.
MSC Osteogenesis
Runt-related transcription factor-2 (Runx-2) is considered a master regulator of early osteogenic differentiation (Fujita et al 2004). In combination with TGF-β, Runx-2 up-regulates the expression of interleukin-11 (IL-11), which reduces adipogenesis (fat formation) and promotes chondrocytic and osteocytic differentiation (Enomoto et al 2004). Runx-2 also promotes the expression of osterix, another important osteogenic inducer. Osterix suppresses chondrogenesis at low concentrations and promotes osteogenesis at high concentrations (Tominaga et al 2009).
Continuous exposure of BMSCs or ADSCs to ligands for the glucocorticoid receptor (e.g., dexamethasone)and/or the vitamin D receptor (e.g., 1,25 dihydroxyvitamin D3), plus ascorbic acid and β-glycerophosphate induces them to produce mineralized extracellular matrix within three weeks (Gimble et al 2008).Exposure of MSCs to BMPs and Wnt signaling proteins also results in successful differentiation into osteoblasts (Peng et al 2003; Shea et al 2003; Kang et al 2004a; Luo et al 2004; Peng et al 2004; Si et al 2006; Luu et al 2007; Deng et al 2008; Tang et al 2009). Additionally, magnetic field stimulation and can also stimulate osteogenic differentiation of MSCs (Singh, YashRoy and Hoque 2006).
Several studies have found that ADSCs and BMSCs from humans and other animals show equal osteogenic potential (Zuk et al 2001; Zuk et al 2002; De Ugarte et al 2003b; Winter et al 2003; Cowan et al 2004; Lee et al 2004a; Romanov et al 2005; Wagner et al 2005; Kern et al 2006). However, other studies argue that BMSCs display superior osteogenic potential to ADSCs (Im, Shin and Lee 2005; Sakaguchi et al 2005; Musina et al 2006; Lui et al 2007; Yoshimura et al 2007). Yet another study insists that ADSCs have superior osteogenic potential than BMSCs (Izadpanah et al 2006).
In head-to-head comparisons with other types of MSCs, the osteogenic potential of BMSCs was approximately the same as SMSCs, and only slightly better than periosteum-derived MSCs (Sakaguchi et al 2005). However, in another study SMSCs from healthy donors expressed significantly lower levels of osteogenic markers after induction of osteogenesis (Djouad et al 2005). Another comparison between human umbilical cord perivascular cells (HUCPVCs) and BMSCs found that HUCPVCs had higher osteogenic potential than BMSCs (Baksh, Yao and Tuan 2007). However, other studies compared the gene expression profiles and osteogenic potential of UCSCs and BMSCs not only showed a pronounced expression of osteogenic genes in BMSCs, but also established their superior osteogenic potential in in vitro differentiation assays (Hsieh et al 2010; Majore et al 2011). It is unclear if these two experiments analyzed the same umbilical cord cell populations. MSCs isolated from human umbilical cord blood also showed a distinctly greater osteogenic potential in comparison to BMSCs (Chang et al 2006a). Also human UCSCs show superior osteogenic potential in comparison to chorionic plate-derived MSCs (Kim et al 2011).
MSC Adipogenesis
Adipocytes are specialized cells that store triacylglycerols (fats). MSC differentiation into adipocytes requires the activity of a transcription factor called peroxisome proliferator activator receptor-gamma (PPAR-γ). PPAR-γ regulates the function of many adipocyte specific genes (Rosen 2000), and interacts with members of the CCAAT/enhancer binding protein (C/EBP) family to regulate adipogenesis (Farmer 2005). Osteogenic transcription factor Runx2 inhibits adipogenesis by directly interacting with PPAR-γ (Akune et al 2004).
Adipogenic induction of cultured MSCs requires the use of compounds that increase intracellular levels of the signaling molecule 3’,5’-cyclic adenosine monophosphate (cAMP) such as phosphodiesterase inhibitors (e.g., isobutylmethylxanthine or theophylline), and ligands for the glucocorticoid receptor (e.g., dexamethasone), and PPAR-γ, (i.e., rosiglitazone, which is marketed as the anti-diabetic insulin sensitizer AvandiaTM). Additionally, most adipogenic cocktails also include insulin, and some protocols also include indomethacine (Mosna, Sensebe and Krampera 2010). MSCs exposed to these agents form intracellular droplets composed of neutral lipid and express key adipogenic markers (e.g., adiponectin, fatty acid binding protein, aP2) within three-to-nine days (Gimble et al 2008; Muruganandan, Roman and Sinal 2009).
Head-to-head comparisons of MSCs from varied tissue sources have shown that ADSCs have an adipogenic potential that is superior (Sakaguchi et al 2005; Izadpanah et al 2006; Musina et al 2006; Liu et al 2007; Yoshimura et al 2007; Rider et al 2008) or equal to that of BMSCs (Zuk et al 2001; 2002; De Ugarte et al 2003b; Winter et al 2003; Lee et al 2004a; Romanov et al 2005;Wagner et al 2005; Kern et al 2006). SMSCs also showed an adipogenic potential that was equal to that of ADSCs and superior to that of periosteum-derived MSCs (Sakaguchi et al 2005; Yoshimura et al 2007). Some studies suggest that UCSCs show poor adipogenic ability in comparison to BMSCs and ADSCs (Rebelatto et al 2008; Hsieh et al 2010), but another study found that HUCPVCs had superior adipogenic potential when compared to BMSCs (Bask, Tao and Tuan 2007). Chorionic-plate-derived MSCs showed superior adipogenic potential to UCSCs (Kim et al 2011), but umbilical cord and umbilical cord blood seem to contain more than one MSC population, all of which display different adipogenic potentials (Chang et al 2006b; Kestendjieva et al 2008; Cheong et al 2010; Lu et al 2010; Majore et al 2011).
MSC Muscle Differentiation
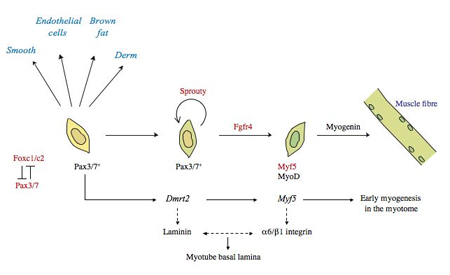
Myogenesis (muscle formation) is regulated by a family of transcription factors known as the myogenic regulatory factors (MRFs). During embryonic development, two basic helix-loop-helix (bHLH) transcription factors, MyoD and Myf5, establish the skeletal muscle lineage and drive myocyte differentiation (Rudnicki et al 1993). Later events in myogenesis that consist of myocyte fusion into myotubes and the synthesis of muscle-specific contractile proteins is associated with the expression of another bHLH transcription factor, myogenin (Hasty et al 1993; Nabeshima et al 1993). Muscle injury activates a muscle stem cell population called satellite cells that recapitulate the MRF expression program (Smith et al 1994; Yablonka-Reuveni and Rivera 1994; Cornelison and Wold 1997; Cooper et al 1999).
Many different types of MSCs can form skeletal, smooth and cardiac muscle. Maintaining MSCs in 10%-20% serum causes them to express smooth muscle markers like α-smooth muscle actin (Abedin, Tintut and Demer 2004; Gimble et al 2008). When transplanted in vitro, MSCs make smooth muscle rather easily (Galmiche et al 1993; Wakitani, Saito and Caplan 1995; Prockop et al 1997; Ferrari et al 1998; Pittenger et al 1999; Caplan and Bruder 2001; Jiang et al 2002).
Exposing MSCs to low serum concentrations or horse serum leads to the expression of skeletal muscle markers such as myogenin and the formation of multi-nuclear myotubes. However, MSCs do not differentiate into mature, skeletal muscles as readily as they do smooth muscles, and the culture conditions under which the cells are grown seem to be extremely important. Co-culturing BMSCs (Lee, Kosinski and Kemp 2005; Beier et al 2011) or ADSCs (Di Rocco et al 2006) with skeletal muscles can induce myotube formation and the expression of myogenic genes by MSCs. The efficiency of skeletal muscle formation with this procedure is almost doubled by exposing MSCs to the chromatin remodeling reagent trichostatin A (Collins-Hooper et al; 2011). Incubation of MSCs with conditioned medium prepared from chemically damaged, but not undamaged, muscle cells also induces MSC myotube formation and expression of MyoD (Santa Maria, Rojas and Minguell; 2004). Treatment of MSCs with particular molecules such as Galectin-1 (Chan et al 2006), TWEAK (Gigenrath et al 2006) and 5-azacytidine (Kocaefe et al 2010; Natasuke et al 2010) can also induce myogenesis, as can hypoxic preconditioning (Leroux et al 2010).
Dezawa and colleagues have published a protocol for differentiating BMSCs into skeletal muscle. They treated mouse BMSCs for three days with a mixture of bFGF, forskolin, which is known to increase intracellular concentrations of cAMP, platelet-derived growth factor and neuregulin. After the three-day culture period, they transfected the cells with a plasmid that encoded the intracellular domain of the Notch receptor, and selected only those cells that had successfully taken up the plasmid. To augment the ability of the remaining cells to form myotubes, they exposed the cells to either 2% horse serum or ITS (insulin-transferrin-selenite) in serum-free medium. Both of these media promoted myogenic differentiation of MSCs to myoblasts that formed myotubes, and were able to integrate into existing muscle and repair muscle in mdx mice (Dezawa et al 2005). mdx Mice harbor a loss-of-function mutation in the gene that encodes the dystrophin protein, which, in humans, is defective in individuals who are afflicted with Duchenne Muscular Dystrophy (Muntoni, Torelli and Ferlini 2003). Therefore, even though it shows a relatively mild phenotype, the mdx mouse is a model system for muscular dystrophy (Sicinski et al 1998).
Treatment of MSCs with a drug called 5-azacytidine directs them to transdifferentiate into cells that resemble cardiomyocytes (heart muscle cells). In cells, 5-azacytidine is incorporated into DNA where it inhibits DNA methylation, and DNA hypomethylation leads to activation of particular genes (Christman 2002). Treatment of BMSCs (Fukuda 2001; Shim et al 2004; Xu et al 2004; Antonitsis et al 2007; 2008), ADSCs (Rangappa et al 2003b; Lee et al 2009) or UCSCs (Cheng et al 2003) with 5-azacytidine drives them to form cells that have a fibroblast-like morphology, synchronously beat, and express many cardiac-specific genes like troponin T, atrial natriuretic protein (ANP), GATA-4, Nkx2.5, TEF-1, and MEF-2C (Fukuda 2001; 2002; Yang et al 2012). Some work has even shown that these differentiated MSCs respond to adrenergic and muscarinic stimulation (Fukuda 2002), and can integrate into the heart of a laboratory animal and form functional connections with native cardiomyocytes (Hattan et al 2005).
MSCs can also be converted into cardiomyocytes by being co-cultured with living (Rangappa et al 2003a; Yoon et al 2005b; Arminan et al 2009; Peran et al 2010) or apoptotic cardiomyocytes (He et al 2010). Also treatment with particular growth factors, such as BMP-2, Fibroblast growth factor -2 (FGF-2) and IGF-1 (Yoon et al 2005a; Bartunek et al 2007; Hahn et al 2008), can push MSCs to become cardiomyocytes, as can transfection with particular genes like Wnt-11 (He et al 2011), GATA-4 (Li et al 2011), or a combination of GATA-4 and Nkx2.5 (Gao, Tan and Wang 2011). Some controversy exists over cardiomyocyte-induced MSCs, since some studies suggest that differentiated MSCs retain their stromal phenotypes and are, at best, only immature cardiomyocytes (Gallo et al 2007; Rose et al 2008).
Because MSC populations tend to form smooth muscle rather readily, there have been few head-to-head comparisons of the efficiency of smooth muscle formation in distinct MSC populations.
Comparisons of the ability of various MSC populations to differentiate into skeletal muscles include in vitro differentiation of MSCs from bone marrow, spleen, thymus, and liver. This study showed that BMSCs, liver- and thymus-derived MSCs all made skeletal muscle in culture, but splenic-derived MSCs did not (Gornostaeva, Rzhaninova and Gol’dstein 2006). Comparisons of the in vivo ability of BMSCs, ADSCs, and SMSCs to form skeletal muscle when implanted showed that ADSCs had the greatest ability to integrate into existing muscles (de la Garza-Rodea et al 2011).
Interestingly, a small fraction of BMSCs can form myotubes and integrate into existing muscle when injected into laboratory animals, whether that muscle is damaged or not (Ferrari et al 1998), a characteristic also shared by SMSCs (De Bari et al 2003). However, when UCSCs were injected into the tail vein of mdx mice, the cells were able to integrate into the muscle but unable to differentiate in vivo into mature, skeletal muscles (Vieira et al 2010; Zucconi et al 2011). Different MSCs show varying efficiencies of cardiomyocyte differentiation. UCSCs, for example, show particularly low transdifferentiation rates (Martin-Rendon et al 2008). ADSCs, however, transdifferentiate into cardiomyocytes with the highest efficiency (Zhu et al 2008; Tobita, Orbay and Mizuno 2011; Paul et al 2011;Yong et al 2012). In fact, when grown in a semisolid methycellulose medium enriched with growth factors, ADSCs spontaneously form beating ventricular- and atrial-like cardiomyocytes (Planat-Benard et al 2004). This makes ADSCs an attractive source of material for cardiac regenerative therapies.
MSCs and Tooth Formation
Tooth formation results from a complex set of interactions between the overlying stomadial epithelium and underlying mesenchymal cells. Dental mesenchymal cells develop from neural crest cells derived from midbrain and hindbrain cranial neural crest cells. In mice, these two cell populations are in place by day 8.5 (E8.5) and by day 10.5 (E10.5) tooth-forming sites and tooth types are determined. At E11.5, a localized thickening of the dental epithelium that results from cell shape changes forms the “dental placode.” Between E12.5-E13.5, the dental placode proliferates and invaginates to form the epithelial bud around which mesenchymal cells condense (Peters and Bailing 1999). At E14.5, the cap stage, the epithelial component of the developing tooth folds and forms a transient cluster of non-dividing cells called the “enamel knot.” The enamel knot is a signaling center that produces many powerful growth factors, including Sonic hedgehog (Shh), BMP-2, BMP-4, BMP-7, FGF-4 and FGF-9 (Thesleff and Mikkola 2002). The cap stage is followed by the bell stage, and at this time the epithelially-derived ameloblasts and the mesenchymally-derived odontoblasts differentiate. The ameloblasts form enamel and the odontoblasts produce the dentine. MSCs also generate the alveolar bone that forms the sockets for the teeth. Human tooth development occurs in a very similar fashion (Zhang et al 2005).
In adult animals, dentinal repair results from odontoblasts that differentiate from a precursor cell population that resides in dental pulp tissue. These dental pulp stem cells (DPSCs) have been isolated from adult human teeth (Gronthos et al 2002). In culture, DPSCs show robust growth and a high proliferation rate and, even after extensive subculturing, have the ability to form a dentin/mineralized complex with a mineralized matrix when grafted into the dorsal surface of immunocompromised mice (Gronthos, et al 2002; Batouli et al 2003). In a rabbit model of tooth regeneration, DPSCs are able to support the formation of functional teeth (Hung et al 2011), and in mouse and dog models, DPSCs regenerated alveolar tooth socket bone in the jaw (Yamada et al 2010; 2011; Ito et al 2011).
Four other dental-associated, MSC-like stem cell populations have been isolated and characterized. The first of these, stem cells from human exfoliated deciduous teeth (SHED), like DPSCs, have many similarities to MSCs. However, SHEDs differ from DPSCs in that they have a higher proliferation rate and can differentiate into odontoblasts, which form a dentin-pulp-like structure without the mineralized matrix, but not ameloblasts (Miura et al 2003). Transplantation experiments have established that SHEDs can make vascularized bone and endothelial cells, and when implanted into the jaws of laboratory animals SHEDs can effectively regenerate jaw bone (Cordeiro et al 2008; Nakamura et al 2009; Yamada et al 2010; 2011; Ito et al 2011). The second cell population, periodontal ligament stem cells (PDLSCs), expresses a subset of neural crest cell and MSC markers (Seo et al 2004; Nagatomo et al 2006; Gay et al 2007; Fujita et al 2007; Coura et al 2008; Huang et al 2009), and shows some ability to repair periodontium (Seo 2004; Grimm et al 2011). The third population, stem cells from apical papillae (SCAP) readily makes dentin-pulp-like complexes and expresses several neuronal markers (Sonoyama et al 2006; 2008). The fourth stem population, dental follicle precursor cells (DFPCs), form fibrous and rigid tissue when transplanted into laboratory animals but not dentin, cementum or bone (Morsczecket al 2005; 2008).
In a head-to-head comparison of the ability of DPSCs and ADSCs to replace teeth in a rabbit model, the teeth produced by ADSCs were very similar to those generated by DPSCs. Both sets of replacement teeth were living teeth with nerves and vascular systems, but the ADSCs grew at faster rate and were more resistant to senescence (Hung et al 2011). BMSCs, like DPSCs, are also able to form calcified deposits in vitro (Gronthos et al 2000). Likewise, gene microarray analyses of these two stem cell populations show similar levels of expression for more than 4000 genes, with only a few differences (Shi, Robey and Gronthos 2001). Head-to-head comparisons of BMSCs, DPSCs, and SHEDs have shown that these stem cells have an equivalent the ability to regenerate alveolar tooth socket bone in the jaws of laboratory animals (Yamada et al 2010; 2011; Ito et al 2011). Comparison of BMSCs and SHED gene expression profiles by means of DNA microarray and real-time reverse transcriptase polymerase chain reaction has shown that 2753 genes in SHEDs show a more than two-fold difference in expression level in comparison to BMSCs. The genes that show the greatest differences in expression in SHEDs are those involved in BMP signaling, and the protein kinase A (PKA), c-Jun-N-terminal kinase (JNK), and apoptosis signaling-regulating kinase-1 (ASK-1) signaling cascades. Therefore SHEDs have specific characteristics that differ from BMSCs, and the osteogenic and odontogenic differentiation of SHEDs and BMSCs are probably regulated by different mechanisms (Hara et al 2009).
BMSCs can probably serve as a source for dental regenerative treatments, but the faster growth rates and easier isolation of ADSCs probably makes them a superior choice.
MSC Neural Differentiation
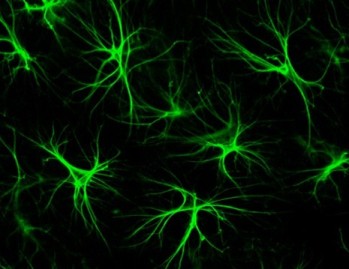
To date, neural differentiation of MSCs remains controversial, since many stem cell biologists think that the neuron-like cells formed by MSCs after neural induction do not represent true neurons. However, protocols have been published for converting MSCs into specific types of neurons. One method (Tropel et al 2006) cultures MSCs at low density (3,000 cells / cm2) on poly-lysine-coated plates for seven days in low-glucose DMEM, 10% fetal calf serum, glutamine (2mM), and bFGF (25ng/mL). A second protocol incubates MSCs with bFGF (5ng / mL) for 24 hours, followed by complete medium substitution with DMEM, N2 supplement, butylated-hydroxyanisole, KCl, valproic acid, and forskolin (Krampera et al 2007; Anghileri et al 2008). When subjected to either protocol, MSCs show dramatic morphological changes after 24-48 hours. They begin to sprout long branches and axon-like structures. Molecularly, neurally induced MSCs up-regulate synthesis of the neuron-specific intermediate filament nestin, which is typically only made by dividing neurons and disappears from terminally differentiated neurons (Michalczyk and Ziman 2005). Neurally induced MSCs also initiate expression of several neuronal and glial markers that include light neurofilament (NF-L), β-tubulin III (β3-tub), peripheral myelin protein-22 (PMP-22), glial fibrillary acidic protein (GFAP), and NeuN or neuronal nuclear antigen (Krampera et al 2007). They also express functional neuronal receptors and pharmacologically sensitive voltage-gated calcium channels (Wislet-Gendebien et al 2005; Tropel et al 2006). Unfortunately, MSC neuronal induction is reversible, and as soon as neural induction ceases MSCs revert back to their ground state. Interestingly, co-culturing neutrally induced MSCs with Schwann cells locks the neutrally induced MSCs in their neuronal state (Krampera et al 2007).
Despite reports that MSCs can be differentiated into functional neurons, several studies have failed to recapitulate these results (Scuteri et al 2010). Time-lapse photography of rat BMSCs that had undergone neural induction showed that instead of extending neurites, the cells merely shrunk and retracted their cell extensions so that only two extensions remained. This was interpreted to be a response to toxic or stressful conditions, and treatment of MSCs with chemicals and conditions known to stress cells (extremes of pH, high-molarity NaCl or detergents) produced similar “pseudoneuronal” morphology and increased MSC staining for neuronal markers. Strangely, pretreatment of MSCs with cycloheximide (an antibiotic that inhibits translation) failed to abrogate this response, suggesting that no new gene expression is required for cells to assume this pseudoneuronal morphology. These findings suggest that neural induction of MSCs in culture is largely an artifact (Lu, Blesch and Tuszynski 2004). Other studies have implanted MSCs into the brains of laboratory animals in the hope that a neural environment can induce neuronal differentiation in MSCs, but the implanted cells showed a spherical morphology with few extensions and connections with other cells (Zhao et al 2002).
Despite these negative results, genetic engineering of MSCs with the intracellular domain of Notch (Dezawa et al 2004; Xu et al 2010), neurogenin-1 (Kim et al 2008), neurotrophin-3 after retinoic acid pretreatment (Zhang et al 2006), siNRSF (Yang et al 2008) and brain-derived neurotrophic factor (Lim et al 2011), have all successfully transdifferentiated MSCs into functional neurons. Furthermore, MSC treatment with various combinations of growth factors (Long et al 2005; Bae et al 2011; Trzaska and Rameshwar 2011), signaling molecules (Kondo et al 2011) and small molecules (Wang et al 2011) have also transdifferentiated MSCs into neurons, and in some cases into dopaminergic neurons. Finally, sequential analysis of gene expression (SAGE) and microRNA expression profiles of MSCs before and after neural induction have shown high level expression of several neural specific genes that are not expressed in MSCs before neural induction. Also cell the expression of reprogramming factors like Oct4, Klf4, and c-Myc are modulated during differentiation (Crobu et al 2011).
With respect to MSC neuronal differentiation, BMSCs have definitely received the most attention. However, other types of MSCs have the capacity to form neuron-like cells (Chen, He and Zhang 2009; Chang et al 2010; Jiang et al 2010; Lim et al 2010). To date there have been few head-to-head comparisons of the efficiency of neural induction between distinct MSC populations, and this is probably a function of the variability of MSC neural induction. One study found that neural induction of UCSCs and BMSCs produces dopaminergic neurons with roughly equal efficiencies (Datta et al 2011).
Also, there are few comparisons with dentally-derived MSCs, but these cells descend from neural crest cells. Consequently, they demonstrate more neural properties than other types of MSCs (Karaoz et al 2011). Such MSCs begin with more neural characteristics, and, therefore, neural differentiation of dental-derived MSCs probably requires fewer molecular steps (Nourbakhsh et al 2011).
Conclusion
Are BMSCs significantly different or relatively similar to MSCs from other tissue sources? The extensive research on BMSCs has provided a wealth of data that we can use for comparison with other MSCs. Work on MCSs from other tissues strongly suggests that genuine similarities exist between BMSCs and other types of MSCs. All these MSCs, with a few exceptions, display roughly the same set of cell surface proteins (De Ugarte et al 2003a; Musina, Bekchanova and Sukhikh 2005). For the most part, clonal differences in specific MSC populations notwithstanding (Zuk et al 2002; Guilak et al 2006), can differentiate into osteocytes, chondrocytes, or adipocytes (Pittenger et al 1999; Pontos et al 2006), and BMSCs and ADSCs utilize common pathways to differentiate into these distinct cell types (Liu et al 2007). They also express a common core of genes and proteins that distinguish them from other cell types.
Despite these similarities, there are also some stark differences between various MSCs from assorted tissues. First of all, the efficiencies with which these different MSC populations differentiate into osteocytes, chondrocytes, and adipocytes widely differ. Secondly, even though BMSCs and ADSCs use a set of common genes for early differentiation into all three lineages, they recruit different sets of genes for later differentiation and maturation into fully differentiated cells (Liu et al 2007; Kim and Im 2010). Thirdly, varied MSC populations differ with regards to their stemness. UCSCs share more genes in common with embryonic stem cells than BMSCs, and are, therefore, more primitive. They also express more angiogenesis and growth related genes. On the other hand, the gene expression profiles of BMSCs are much more significantly altered under different culture conditions, and express more osteogenesis genes (Hsieh et al 2010). Fourth, even though MSC populations commonly express a core set of genes(Winter et al 2003; Lee et al 2004a; Djouad et al 2005; Wagner et al 2005; Aranda et al 2009; Jansen et al 2010), gene expression profiles of distinct MSC populations differs substantially. For example, UCSCs and umbilical cord blood-derived MSCs(UBSCs) show remarkable differences in gene expression. Gene expression profiles from UBSCs revealed that genes involved in anatomical structure and multicellular organism development, osteogenesis and the immune system were expressed at high levels. However in UCSCs, genes related to cell adhesion, neurogenesis, morphogenesis, secretion and angiogenesis were more highly expressed (Secco et al 2009). Fifth, even though distinct MSC populations express very similar sets of proteins (Roche et al 2009), there are significant differences (Maurer 2011). Finally, the differentiation requirements for each MSC population differ, and these differences are a result of the signature gene expression profiles of each MSC population.
Thus, MSCs represent a familial cell type, but each distinctive MSC population represents a particular subfamily of this cell type family. While some subfamilies are clearly more closely related to some than others, these MSC subfamilies constitute the constituents that compose the MSC cell type.
References
Abedin M, Tintut Y, Demer LL. Mesenchymal Stem Cells and the Artery Wall. Circulation Research 2004;95:671-676.
Afizah H, Yang Z, Hui JHP, Ouyang HW, Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng 2007;13(4):659-666.
Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 2004;113(6):846-855.
Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galie M, Sbarbati A, Krampera M, Belluzzi O, Bonetti B. Neuronal differentiation potential of human adipose derived mesenchymal stem cells. Stem Cells Dev 2008;17(5):909-916.
Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Papakonstantinou C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact Cardiovasc Thorac Surg 2007;6(5):593-597.
Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Charokopos N, Kalogeridis A, Kouzi-Koliakou K, Kyriakopoulou I, Klonizakis I, Papakonstantinou C. Cardiomyogenic potential of human adult bone marrow mesenchymal stem cells in vitro. Thorac Cardiovasc Surg 2008;56(2):77-82.
Aranda P, Agirre X, Ballestar E, Andreu EJ, Roman-Gomez J, Prieto I, Martin-Subero JI, Cigudosa JC, Siebert R, Esteller M, Prosper F. Epigenetic signatures associated with different levels of differentiation potential in human stem cells. PLoS One 2009;4(11):e7809.
Arminan A, Gandia C, Bartual M, Garcia-Verdugo JM, Lledo E, Mirabet V, Llop M, Barea J, Montero JA, Sepulveda P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev 2009;18(6):907-918.
Awad HA, Halvorsen YD, Gimble JM, Guilak F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng 2003;9(6):1301-1312.
Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004;25(16):3211-3222.
Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med
2011;52(3):401-412.
Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007;25:1384-1392.
Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 2001;268:189-200.
Bartolini P, Vainzof M, Zatz M. Preclinical studies with umbilical cord mesenchymal stromal cells in different animal models for muscular dystrophy. J Biomed Biotechnol 2011;DOI:10.1155/2011/715251.
Bartunek J, Croissant JD, Wijns W, Gofflot S, de Lavareille A, Vanderheyden M, Kaluzhny Y, Mazouz N, Willemsen P, Penicka M, Mathieu M, Homsy C, De Bruyne B, McEntee K, Lee IW, Heyndrickx GR. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium.Am J Physiol Heart Circ Physiol 2007;292(2):H1095-H1104.
Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, Robey PG, Shi S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 2003;82(12):976-981.
Beier JP, Bitto FF, Lange C, Klumpp D, Arkudas A, Bleiziffer O, Boos AM, Horch RE, Kneser U. Myogenic differentiation of mesenchymal stem cells cocultured with primary myoblasts. Cell Biol Int 2011;35(4):397-406.
Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004;22:625–
634.
Boeuf S, Richter W. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Res Ther 2010;1(4):31.
Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 2001;7:259–264.
Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner S, Robinson B, Hanson R. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther 2011;6(4):368-378.
Chang YJ, Shih D, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate Mesenchyme-Lineage Tendencies in Mesenchymal Stem Cells from Human Bone Marrow and Umbilical Cord Blood. Stem Cells 2006a;24:679-685.
Chang YJ, Tseng CP, Hsu LF, Hsieh TB, Hwang SM. Characterization of two populations of mesenchymal progenitor cells in umbilical cord blood. Cell Biol Int 2006b;30(6):495-499.
Chang YJ, Hwang SM, Tseng CP, Cheng FC, Huang SH, Hsu LF, Hsu LW, Tsai MS. Isolation of mesenchymal stem cells with neurogenic potential from the mesoderm of the amniotic membrane. Cells Tissues Organs 2010;192(2):93-105.
Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther 2010;21(9):1045-1056.
Chen L, He DM, Zhang Y. The differentiation of human placenta-derived mesenchymal stem cells into dopaminergic cells in vitro. Cell Mol Biol Lett 2009;14(3):528-536.
Cheng F, Zou P, Yang H, Yu Z, Zhong Z. Induced differentiation of human cord blood mesenchymal stem/progenitor cells into cardiomyocyte-like cells in vitro. J Huazhong Univ Sci Technolog Med Sci 2003;23(2):154-157.
Cheong HH, Masilamani J, Phan TT, Chan SY. Cord lining progenitor cells: potential in vitro adipogenesis model.Int J Obes (Lond) 2010;34(11):1625-1633.
Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 2002;21(35): 5483-5495.
Collins-Hooper H, Luke G, Cranfield M, Otto WR, Ray S, Patel K. Efficient myogenic reprogramming of adult white fat stem cells and bone marrow stem cells by freshly isolated skeletal muscle fibers. Transl Res 2011;158(6):334-343.
Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci 1999;112 (Pt 17):2895-2901.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nor JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 2008;34(8):962-969.
Cornelison DD, Wold BJ.Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol
1997;191(2):270-283.
Coura GS, Garcez RC, de Aguiar CB, Alvarez-Silva M, Magini RS, Trentin AG. Human periodontal ligament: a niche of neural crest stem cells. J Periodontal Res 2008;43(5):531-536.
Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical size mouse calvarial defects.Nat Biotechnol 2004;22(5):560-567.
Crobu F, Latini V, Marongiu MF, Sogos V, Scintu F, Porcu S, Casu C, Badiali M, Sanna A, Manchinu MF, Ristaldi MS. Differentiation of single cell derived human mesenchymal stem cells into cells with a neuronal phenotype: RNA and microRNA expression profile. Mol Biol Rep 2011;DOI:10.1007/s11033-011-1180-1189.
Datta I, Mishra S, Mohanty L, Pulikkot S, Joshi PG. Neuronal plasticity of human Wharton’s jelly mesenchymal stromal cells to the dopaminergic cell type
compared with human bone marrow mesenchymal stromal cells. Cytotherapy 2011;13(8):918-932.
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane.Arthritis Rheum 2001;44(8):1928-1942.
De Bari C, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol 2003;160(6):909-918.
de la Garza-Rodea AS, van der Velde-van Dijke L, Boersma H, Goncalves MA, van Bekkum DW, de Vries AA, Knaan-Shanzer S. Myogenic properties of human mesenchymal stem cells derived from three different sources. Cell Transplant 2011;DOI:http://dx.doi.org/10.3727/096368911X580554.
De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, Benhaim P, Hedrick MH, Fraser JK. Differential expression of stem cell mobilization associated molecules on multi-lineage cells from adipose tissue and bone marrow.Immunol Lett 2003a;89(2-3):267-270.
De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003b;174(3):101-109.
Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC. Regulation of osteogenic differentiation during skeletal development.Front Biosci 2008;13:2001-2021.
Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest 2004;113(12):1701-1710.
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005;309(5732):314-317.
Di Rocco G, Iachininoto MG, Tritarelli A, Straino S, Zacheo A, Germani A, Crea F, Capogrossi MC. Myogenic potential of adipose-tissue-derived cells.J Cell Sci 2006;119(Pt 14):2945-2952.
Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth
factors and cartilage-derived matrix.Tissue Eng2010;16(2):523-533.
Djouad F, Bony C, Haupl T, Uze G, Lahlou N, Louis-Plence P, Apparailly F, Canovas F, Reme T, Sany J, Jorgensen C, Noel D. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells.Arthritis Res Ther 2005;7(6):R1304-R1315.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315-317.
Enomoto H, Furuichi T, Zanma A, Yamana K, Yoshida C, Sumitani S, Yamamoto H, Enomoto-Iwamoto M, Iwamoto M, Komori T. Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. J Cell Sci 2004;117(Pt 3):417-425.
Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 2002;290:763-769.
Estes BT, Wu AW, Guilak F. Potent Induction of Chondrocytic Differentiation of Human Adipose-Derived Adult Stem Cells by Bone Morphogenetic Protein 6. Arthritis Rheum2006;54(4):1222-1232.
Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc 2010;5(7):1294–1311.
Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E. Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J
2011;22(2):91-98.
Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond) 2005;29(Suppl 1):S13-S16.
Ferrari G, Cusella-De Angelis G, Coletta M., Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle Regeneration by Bone Marrow-Derived Myogenic Progenitors. Science 1998;279:1528–1530.
Fickert S, Fiedler J, Brenner RE. Identification, quantification and isolation of mesenchymal progenitor cells from osteoarthritic synovium by fluorescence automated cell sorting. Osteoarthritis Cartilage 2003;11:790–800.
Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res 2009;27(12):1675-1680.
Fujita T, Azuma Y, Fukuyama R, Hattori Y, Yoshida C, Koida M, Ogita K, Komori T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol 2004;166(1):85-95.
Fujita T, Iwata T, Shiba H, Igarashi A, Hirata R, Takeda K, Mizuno N, Tsuji K, Kawaguchi H, Kato Y, Kurihara H. Identification of marker genes distinguishing human periodontal ligament cells from human mesenchymal stem cells and human gingival fibroblasts.J Periodontal Res 2007;42(3):283-286.
Fukuda K. Development of regenerative cardiomyocytes from mesenchymal stem cells for cardiovascular tissue engineering. Artif Organs 2001;25(3):187-193.
Fukuda K. Molecular characterized of regenerated cardiomyocytes derived from adult mesenchymal stem cells. Congenit Anom (Kyoto) 2002;42(1):1-9.
Gallo MP, Ramella R, Alloatti G, Penna C, Pagliaro P, Marcantoni A, Bonafe F, Losano G, Levi R. Limited plasticity of mesenchymal stem cells cocultured with adult cardiomyocytes.J Cell Biochem 2007;100(1):86-99.
Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood 1993;82:66–76.
Gao XR, Tan YZ, Wang HJ. Overexpression of Csx/Nkx2.5 and GATA-4 enhances the efficacy of mesenchymal stem cell transplantation after myocardial infarction.Circ J 2011;75(11):2683-2691.
Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells.Orthod Craniofac Res 2007;10(3):149-160.
Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362-369.
Gimble JM, Guilak F, Nuttall ME, Sathishkumar S, Vidal M, Bunnell BA. In vitro differentiation potential of mesenchymal stem cells. Trans Med Hemother 2008;35:228-238.
Girgenrath M, Weng S, Kostek CA, Browning B, Wang M, Brown SA, Winkles JA, Michaelson JS, Allaire N, Schneider P, Scott ML, Hsu YM, Yagita H, Flavell RA, Miller JB, Burkly LC, Zheng TS. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J 2006;25(24):5826-5839.
Golding MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem 2006;97:33-44.
Gornostaeva SN, Rzhaninova AA, Gol’dstein DV. Myogenesis in hemopoietic tissue mesenchymal stem cell culture. Bull Exp Biol Med 2006;141(4):493-499.
Grimm WD, Dannan A, Becher S, Gassmann G, Arnold W, Varga G, Dittmar T. The ability of human periodontium-derived stem cells to regenerate periodontal tissues: a preliminary in vivo investigation.Int J Periodontics Restorative Dent 2011;31(6):e94-e101.
Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood 1994;84(12):4164-4173.
Gronthos S, Zannettino AC, Graves SE, Ohta S, Hay SJ, Simmons PJ. Differential cell surface expression of the STRO-1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells.J Bone Miner Res 1999;14(1):47-56.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo.Proc Natl Acad Sci U S A 2000;97(25):13625-13630.
Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM.Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 2001;189(1):54-63.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res 2002;81(8):531-535.
Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow.J Cell Sci 2003;116(Pt 9):1827-1835.
Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM. Clonal Analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol 2006;206:229-237.
Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction.J Am Coll Cardiol 2008;51(9):933-943.
Hara K, Yamada Y, Nakamura S, Umemura E, Ito K, Ueda M. Potential characteristics of stem cells from human exfoliated deciduous teeth compared with bone marrow-derived mesenchymal stem cells for mineralized tissue-forming cell biology. J Endod 2011;37(12):1647-1652.
Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 1993;364(6437):501-506.
Hattan N, Kawaguchi H, Ando K, Kuwabara E, Fujita J, Murata M, Suematsu M, Mori H, Fukuda K. Purified cardiomyocytes from bone marrow mesenchymal stem cells produce stable intracardiac grafts in mice. Cardiovasc Res 2005;65(2):334-344.
He XQ, Chen MS, Li SH, Liu SM, Zhong Y, McDonald Kinkaid HY, Lu WY, Weisel RD, Li RK. Co-culture with cardiomyocytes enhanced the myogenic conversion of mesenchymal stromal cells in a dose-dependent manner. Mol Cell Biochem 2010;339(1-2):89-98.
He Z, Li H, Zuo S, Pasha Z, Wang Y, Yang Y, Jiang W, Ashraf M, Xu M. Transduction of Wnt11 promotes mesen chymal stem cell transdifferentiation into cardiac phenotypes.Stem Cells Dev 2011;20(10):1771-1778.
Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenesis potential adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol 2007;211:682-691.
Hsieh JY, Fu YS, Chang SJ, Tsuang YH, Wang HW. Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton’s jelly of umbilical cord. Stem Cells Dev2010;19(12):1895-1910.
Hu Y, Liao L, Wang Q, Ma L, Ma G, Jiang X, Zhao RC. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J Lab Clin Med 2003;141:342–349.
Huang JL, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res 2005;23:1383-1389.
Huang CY, Pelaez D, Dominguez-Bendala J, Garcia-Godoy F, Cheung HS. Plasticity of stem cells derived from adult periodontal ligament. Regen Med 2009;4(6):809-821.
Hung CN, Mar K, Chang HC, Chiang YL, Hu HY, Lai CC, Chu RM, Ma CM. A comparison between adipose tissue and dental pulp as sources of MSCs for tooth regeneration. Biomaterials 2011;32(29):6995-7005.
Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy2004;6:543–553.
Im GI, Kim DY, Shin JH, Hyun CW, Cho WH. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg Br 2001;83:289-294.
Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage2005;13(10):845-853.
Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun 2004;320(3):914-919.
Ito K, Yamada Y, Nakamura S, Ueda M. Osteogenic potential of effective bone engineering using dental pulp stem cells, bone marrow stem cells, and periosteal cells for osseointegration of dental implants. Int J Oral Maxillofac Implants 2011;26(5):947-954.
Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006;99(5):1285-1297.
Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adiposetissue-derivedstemcells using bFGF and forskolin. BMC Cell Biol 2010;11:25
Jansen BJ, Gilissen C, Roelofs H, Schaap-Oziemlak A, Veltman JA, Raymakers RA, Jansen JH, Kogler G, Figdor CG, Torensma R, Adema GJ. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev 2010;19(4):481-490.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49.
Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 1998; 238:265-272.
Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 2002;46(12):3349-3360.
Kadar K, Kiraly M, Porcsalmy B, Molnar B, Racz GZ, Blazsek J, Kallo K, Szabo EL, Gera I, Gerber G, Varga G. Differentiation potential of stem cells from human dental origin – promise for tissue engineering. J Physiol Pharmacol 2009;60 Suppl 7:167-175.
Kadiyala S, Young RG, Thiede MA, Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant 1997;6:125–134.
Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther 2004a;11(17):1312-1320.
Kang TJ, Yeom JE, Lee HJ, Rho SH, Han H, Chae GT. Growth kinetics of human mesenchymal stem cells from bone marrow and umbilical cord blood. Acta Haematol 2004b;112(4):230-233.
Karaoz E, Demircan PC, Sağlam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol 2011;136(4):455-473.
Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 2005;23:412–423.
Kern S, Eicher H, Stoeve J, Kluter H, Bieback K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006;24:1294–1301.
Kestendjieva S, Kyurkchiev D, Tsvetkova G, Mehandjiev T, Dimitrov A, Nikolov A, Kyurkchiev S. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol Int 2008;32(7):724-732.
Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells 2008;26(9):2217-2228.
Kim HJ, Im GI. The effects of ERK1/2 inhibitor on the chondrogenesis of bone marrow- and adipose tissue-derived multipotent mesenchymal stromal cells. Tissue Eng Part A 2010;16(3):851-860.
Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res 2009;27: 612-619.
Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, Cha DH, Yoon TK, Kim GJ. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res 2011;346(1):53-64.
Kocaefe C, Balci D, Hayta BB, Can A. Reprogramming of human umbilical cord stromal mesenchymal stem cells for myogenic differentiation and muscle repair. Stem Cell Rev 2010;6(4):512-522.
Kondo T, Matsuoka AJ, Shimomura A, Koehler KR, Chan RJ, Miller JM, Srour EF, Hashino E. Wnt signaling promotes neuronal differentiation from mesenchymal stem cells through activation of Tlx3. Stem Cells 2011;29(5):836-846.
Krampera M, Marconi S, Pasini A, Galie M, Rigotti G, Mosna F, Tinelli M, Lovato L, Anghileri E, Andreini A, Pizzolo G, Sbarbati A, Bonetti B. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone 2007;40(2):382-390.
Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol 2001;153:1133–1140.
Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 2004a;14(4-6):311-324.
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004b;103:1669-1675.
Lee JH, Kosinski PA, Kemp DM. Contribution of human bone marrow stem cells to individual skeletal myotubes followed by myogenic gene activation. Exp Cell Res 2005;307(1):174-182.
Lee WC, Sepulveda JL, Rubin JP, Marra KG. Cardiomyogenic differentiation potential of human adipose precursor cells. Int J Cardiol 2009;133(3):399-401.
Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamaziere JM, Dufourcq P, Couffinhal T, Duplaa C. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther 2010;18(8):1545-1552.
Li H, Zuo S, Pasha Z, Yu B, He Z, Wang Y, Yang X, Ashraf M, Xu M. GATA-4 promotes myocardial transdifferentiation of mesenchymal stromal cells via upregulating IGFBP-4.Cytotherapy 2011;13(9):1057-1065.
Lim JH, Boozer L, Mariani CL, Piedrahita JA, Olby NJ. Generation and characterization of neurospheres from canine adiposetissue-derived stromal cells. Cell Reprogram 2010;12(4):417-425.
Lim JY, Park SI, Kim SM, Jun JA, Oh JH, Ryu CH, Jeong CH, Park SH, Park SA, Oh W, Chang JW, Jeun SS. Neural Differentiation of Brain-derived Neurotrophic Factor-expressing Human Umbilical Cord Blood-derived Mesenchymal Stem Cells in Culture via TrkB-mediated ERK and β-catenin Phosphorylation and following Transplantation into the Developing Brain. Cell Transplant 2011;DOI:10.3727/096368910X557236:1-32.
Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 2007;25(3):750-760.
Long X, Olszewski M, Huang W, Kletzel M. Neural cell differentiation in vitro from adult human bone marrow mesenchymal stem cells. Stem Cells Dev 2005;14(1):65-69.
Lovati AB, Corradetti B, Consiglio AL, Recordati C, Bonacina E, Bizzaro D, Cremonesi F. Comparison of equine bone marrow-, umbilical cord matrix and amniotic fluid-derived progenitor cells. Vet Res Commun 2011;35:103-121.
Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: Differentiation, transdifferentiation or artifact? J Neurosci Res 2004;77(2):174-191.
Lu X, Alshemali S, de Wynter EA, Dickinson AM. Mesenchymal stem cells from CD34(-) human umbilical cord blood. Transfus Med 2010;20(3):178-184.
Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem 2004;279(53):55958-55968.
Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, He TC. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res 2007;25(5):665-677.
Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 1998;4:415-428.
Mafi P, Hindocha S, Mafi R, Griffin M, Khan WS. Adult mesenchymal stem cells and cell surface characterization – a systematic review of the literature. Open Orthop J 2011;5(Suppl 2):253-260.
Majore I, Moretti P, Stahl F, Hass R, Kasper C. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev 2011;7(1):17-31.
Martin-Rendon E, Sweeney D, Lu F, Girdlestone J, Navarrete C, Watt SM. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang 2008;95(2):137-148.
Maurer MH. Proteomic definitions of mesenchymal stem cells. Stem Cells Int 2011;DOI:10.4061/2011/7042562011.
Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol 2005;20(2):665-671.
Mitchell KE, Weiss ML, Mitchel BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells 2003;21:50-60.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807-5812.
Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, Matsuzaki Y. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun 2009; 379(4):1114-1119.
Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 2005;24(2):155-165.
Morsczeck C, Schmalz G, Reichert TE, Vollner F, Galler K, Driemel O. Somatic stem cells for regenerative dentistry. Clin Oral Investig 2008;12(2):113-118.
Mosna F, Sensebe L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: A user’s guide. Stem Cells Dev 2010;19(10):1449-1470.
Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet neurology 2003;2(12):731–740.
Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 2009;66(2):236-253.
Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, Nabeshima Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 1993;364(6437):532-535.
Nagatomo K, Komaki M, Sekiya I, Sakaguchi Y, Noguchi K, Oda S, Muneta T, Ishikawa I. Stem cell properties of human periodontal ligament cells. J Periodontal Res 2006;41(4):303-310.
Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell 2008;2(4):392-403.
Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod 2009;35(11):1536-1542.
Nakatsuka R, Nozaki T, Uemura Y, Matsuoka Y, Sasaki Y, Shinohara M, Ohura K, Sonoda Y. 5-Aza-2′-deoxycytidine treatment induces skeletal myogenic differentiation of mouse dental pulp stem cells. Arch Oral Biol 2010;55(5):350-357.
Niemeyer P, Kornacker M, Mehlhorn A, Seckinger A, Vohrer J, Schmal H, Kasten P, Eckstein V, Sudkamp NP, Krause U. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng 2007;13(1):111-121
Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehlhorn AT, Pearce S, Kasten P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia the influence of platelet-rich plasma. Biomaterials 2010;31(13):3572-3579.
Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, Umezawa A, Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum 2008;58(2):501-510.
Nourbakhsh N, Soleimani M, Taghipour Z, Karbalaie K, Mousavi SB, Talebi A, Nadali F, Tanhaei S, Kiyani GA, Nematollahi M, Rabiei F, Mardani M, Bahramiyan H, Torabinejad M, Nasr-Esfahani MH, Baharvand H. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth derived stem cells. Int J Dev Biol 2011;55(2):189-195.
Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development 1994;120:177-187.
Paul A, Srivastava S, Chen G, Shum-Tim D, Prakash S. Functional Assessment of Adipose Stem Cells for Xenotransplantation Using Myocardial Infarction Immunocompetent Models: Comparison with Bone Marrow Stem Cells. Cell Biochem Biophys 2011;DOI:10.1007/s12013-011-9323-0.
Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W, Luu HH, Park JY, Haydon RC, He TC. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem 2003;90(6):1149-1165.
Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, Luu HH, Park JK, Li X, Luo J, Montag AG, Haydon RC, He TC. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem 2004;279(31):32941-32949.
Peran M, Marchal JA, Lopez E, Jimenez-Navarro M, Boulaiz H, Rodriguez-Serrano F, Carrillo E, Sanchez-Espin G, de Teresa E, Tosh D, Aranega A. Human cardiac tissue induces transdifferentiation of adult stem cells towards cardiomyocytes. Cytotherapy 2010;12(3):332-337.
Peters H, Bailing R. Teeth: Where and how to make them. Trends Genet 1999;15:59-65.
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Moorman, M. A., Simonetti, D. W., Craig, S., and Marshak, D. R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999;284:143–147.
Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res 2004;94(2):223-229.
Pountos I, Jones E, Tzioupis C, McGonagle D, Giannoudis PV. Growing bone and cartilage. The role of mesenchymal stem cells. J Bone Joint Surg Br 2006;88(4):421-426.
Prockop DJ. Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues. Science 1997; 276:71–74.
Puetzer JL, Petitte JN, Loboa EG. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev 2010;16(4):435-444.
Quirici N, Scavullo C, de Girolamo L, Lopa S, Arrigoni E, Deliliers GL, Brini AT. Anti-L-NGFR and -CD34 monoclonal antibodies identify multipotent mesenchymal stem cells in human adipose tissue. Stem Cells Dev 2010;19(6):915-925.
Rangappa S, Entwistle JW, Wechsler AS, Kresh JY. Cardiomyocyte-mediated contact programs human mesenchymal stem cells to express cardiogenic phenotype. J Thorac Cardiovasc Surg 2003a;126(1):124-132.
Rangappa S, Fen C, :Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg 2003b;75(3):775-779.
Rastegar F, Shenaq D, Huang J, Zhang W, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, Wagner ER, Huang E, Gao Y, Gao JL, Kim SH, Zhou JZ, Bi Y, Su Y, Zhu G, Luo J, Luo X, Qin J, Reid RR, Luu HH, Haydon RC, Deng ZL, He TC. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J Stem Cells 2010;2(4):67-80.
Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med2008;233(7):901-913.
Rider DA, Dombrowski C, Sawyer AA, Ng GH, Leong D, Hutmacher DW, Nurcombe V, Cool SM. Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells 2008;26(6):1598-1608.
Roche S, Delorme B, Oostendorp RA, Barbet R, Caton D, Noel D, Boumediene K, Papadaki HA, Cousin B, Crozet C, Milhavet O, Casteilla L, Hatzfeld J, Jorgensen C, Charbord P, Lehmann S. Comparative proteomic analysis of human mesenchymal and embryonic stem cells: towards the definition of a mesenchymal stem cell proteomic signature. Proteomics 2009;9(2):223-232.
Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med 2005;140(1):138-143.
Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, Keating SC, Parker TG, Backx PH, Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells 2008;26(11):2884-2892.
Rosen ED. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 2000;16:145-171.
Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell1993;75(7):1351-1359.
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stemcells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 2005;52(8):2521-2529.
Santa Maria L, Rojas CV, Minguell JJ. Signals from damaged but not undamaged skeletal muscle induce myogenic differentiation of rat bone-marrow-derived mesenchymal stem cells. Exp Cell Res 2004;300(2):418-426.
Schmitt B, Ringe J, Haupl T, Notter M, Manz R, Burmester GR, Sittinger M, Kaps C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation 2003;71:567-577.
Scuteri A, Miloso M, Foudah D, Orciani M, Cavaletti G, Tredici G. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther 2011;6(2):82-92.
Secco M, Moreira YB, Zucconi E, Vieira NM, Jazedje T, Muotri AR, Okamoto OK, Verjovski-Almeida S, Zatz M. Gene expression profile of mesenchymal stem cells from paired umbilical cord units: cord is different from blood. Stem Cell Rev 2009;5(4):387-401.
Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, Ezura Y, Umezawa A, Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res 2009;27(4):435-441.
Sekiya I, Colter DC, Prockop DJ. BMP-8 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun 2001;284:411-418.
Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA 2002;99:4397-4402.
Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res 2005;320(2):269-276.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004;364(9429):149-155.
Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem 2003;90(6):1112-1127.
Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis.Bone 2001;29(6):532-539.
Shim SW, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun 2004;324(2):481-488.
Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RC, He TC. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells.Mol Cell Biol 2006;26(8):2955-2964.
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 1989;244(4912):1578-1580.
Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991;78(1):55-62.
Simmons PJ, Masinovsky B, Longenecker BM, Berenson R, Torok-Storb B, Gallatin WM. Vascular cell adhesion molecule-1 expressed by bone marrow stromal cells mediates the binding of hematopoietic progenitor cells. Blood 1992;80(2):388-395.
Singh P, YashRoy RC, Hoque M. Augmented bone-matrix formation and osteogenesis under magnetic field stimulation in vivo XRD, TEM and SEM investigations. Indian J Biochem Biophys 2006;43(3):167-172.
Smith CK 2nd, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol 1994;159(2):379-385.
Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 2006;1:e79.
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 2008;34(2):166-171.
Stewart K, Walsh S, Screen J, Jefferiss CM, Chainey J, Jordan GR, Beresford JN. Further characterization of cells expressing STRO-1 in cultures of adult human bone marrow stromal cells. J Bone Miner Res 1999;14(8):1345-1356.
Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 2007;129(7):1377-1388.
Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, Su YX, Jiang W, Tang M, He Y, Wang Y, Chen L, Zuo GW, Shen J, Pan X, Reid RR, Luu HH, Haydon RC, He TC. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med 2009;13(8B):2448-2464.
Thesleff I, Mikkola M. The role of growth factors in tooth development. Int Rev Cytol 2002;217:93-135.
Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med 2011;11(57):160-170.
Tominaga H, Maeda S, Miyoshi H, Miyazono K, Komiya S, Imamura T. Expression of osterix inhibits bone morphogenetic protein-induced chondrogenic differentiation of mesenchymal progenitor cells. J Bone Miner Metab 2009;27(1):36-45.
Tropel P, Platet N, Platel JC, Noel D, Albrieux M, Benabid AL, Berger F. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 2006;24(12):2868-2876.
Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 2008;26:591-599.
Trzaska KA, Rameshwar P. Dopaminergic neuronal differentiation protocol for human mesenchymal stem cells. Methods Mol Biol 2011;698:295-303.
Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 2004;19:1450–1456.
Tsai MS, Hwang SM, Chen KD, Lee YS, Hsu LW, Chang YJ, Wang CN, Peng HH, Chang YL, Chao AS, Chang SD, Lee KD, Wang TH, Wang HS, Soong YK. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. StemCells 2007;25(10):2511-2523.
Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen activated protein kinase and Wnt signaling cross-talk. J Biol Chem 2003;278:41227-41236.
Van Landuyt KB, Jones EA, McGonagle D, Luyten FP, Lories RJ. Flow cytometric characterization of freshly isolated and culture expanded human synovial cell populations in patients with chronic arthritis. Arthritis Res Ther 2010;12(1):R15.
Vidal MA, Robinson SO, Lopez MJ, Paulsen DB, Borkhsenious O, Johnson JR, Moore RM, Gimble JM. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg 2008; 37(8):713-724.
Vieira NM, Zucconi E, Bueno CR Jr, Secco M, Suzuki MF, Bartolini P, Vainzof M, Zatz M. Human multipotent mesenchymal stromal cells from distinct sources show different in vivo potential to differentiate into muscle cells when injected in dystrophic mice. Stem Cell Rev 2010;6(4):560-566.
Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005;33(11):1402-1416.
Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 1994;76;579-592.
Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 1995;18:1417–1426.
Walsh S, Jefferiss C, Stewart K, Jordan GR, Screen J, Beresford JN. Expression of the developmental markers STRO-1 and alkaline phosphatase in cultures of human marrow stromal cells: regulation by fibroblast growth factor (FGF)-2 and relationship to the expression of FGF receptors 1-4. Bone 2000;27(2):185-195.
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s Jelly of the human umbilical cord. Stem Cells 2004;22:1330-1337.
Wang L, Tran I, Sesharedy K, Weiss ML, Detamore MS. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord derived mesenchymal stem cells for cartilage tissue engineering. Tissue Eng 2009;15(8):2259-2266.
Wang Y, He W, Bian H, Liu C, Li S. Small molecule induction of neural-like cells from bone marrow-mesenchymal stem cells. J Cell Biochem 2011;DOI:10.1002/jcb.24021.
Warejcka DJ, Harvey R, Taylor BJ, Young HE, Lucas PA. A population of cells isolated from rat heart capable of differentiating into several mesodermal phenotypes. J Surg Res 1996;62:233–242.
Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol 2010;223:84-93.
Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum 2003;48(2):418-429.
Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells 2005;23(3):392-402.
Wongchuensoontorn C, Liebehenschel N, Schwarz U, Schmelzeisen R, Gutwald R, Ellis E 3rd, Sauerbier S. Application of a new chair-side method for the harvest of mesenchymal stem cells in a patient with nonunion of a fracture of the atrophic mandible–a case report. J Craniomaxillofac Surg 2009;37(3):155-161.
Woods A, Wang G, Beier F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol 2007;213:1-8.
Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult huan bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229(7):623-631.
Xu D, Gechtman Z, Hughes A, Collins A, Dodds R, Cui X, Jolliff e L, Higgins L, Murphy A, Farrell F. Potential involvement of BMP receptor type IB activation in a synergistic effect of chondrogenic promotion between rhTGFbeta3 and rhGDF5 or rhBMP7 in human mesenchymal stem cells. Growth Factors 2006;24:268-278.
Xu H, Miki K, Ishibashi S, Inoue J, Sun L, Endo S, Sekiya I, Muneta T, Inazawa J, Dezawa M, Mizusawa H. Transplantation of neuronal cells induced from human mesenchymal stem cells improves neurological functions after stroke without cell fusion. J Neurosci Res 2010;88(16):3598-3609.
Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol 1994;164(2):588-603.
Yamada Y, Nakamura S, Ito K, Sugito T, Yoshimi R, Nagasaka T, Ueda M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng Part A 2010;16(6):1891-1900.
Yamada Y, Ito K, Nakamura S, Ueda M, Nagasaka T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant 2011;20(7):1003-1013.
Yang Y, Li Y, Lv Y, Zhang S, Chen L, Bai C, Nan X, Yue W, Pei X. NRSF silencing induces neuronal differentiation of human mesenchymal stem cells. Exp Cell Res 2008;314(11-12):2257-2265.
Yang J, Song T, Wu P, Chen Y, Fan X, Chen H, Zhang J, Huang C. Differentiation potential of human mesenchymal stem cells derived from adipose tissue and bone marrow to sinus node-like cells. Mol Med Report 2012;5(1):108-113.
Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 1998;80(12):1745-1757.
Yoon J, Min BG, Kim YH, Shim WJ, Ro YM, Lim DS. Differentiation, engraftment and functional effects of pre-treated mesenchymal stem cells in a rat myocardial infarct model. Acta Cardiol 2005a;60(3):277-284.
Yoon J, Shim WJ, Ro YM, Lim DS. Transdifferentiation of mesenchymal stem cells into cardiomyocytes by direct cell-to-cell contact with neonatal cardiomyocyte but not adult cardiomyocytes. Ann Hematol 2005b;84(11):715-721.
Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 2007;327:449-462.
Young HE, Mancini ML, Wright RP, Smith JC, Black AC Jr, Reagan CR, Lucas PA. Mesenchymal stem cells reside within the connective tissues of many organs. Dev Dyn1995;202:137–144.
Yu G, Wu X, Dietrich MA, Polk P, Scott LK, Ptitsyn AA, Gimble JM. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy 2010;12(4):538-546.
Zhang YD, Chen Z, Song YQ, Liu C, Chen YP. Making a tooth: Growth factors, transcription factors, and stem cells. Cell Res 2005;15(5):301-316.
Zhang W, Zeng YS, Zhang XB, Wang JM, Zhang W, Chen SJ. Combination of adenoviral vector-mediated neurotrophin-3 gene transfer and retinoic acid promotes adult bone marrow cells to differentiate into neuronal phenotypes. Neurosci Lett 2006;408(2):98-103.
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 2002;174(1):11-20.
Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct 2008;26(6):664-675.
Zucconi E, Vieira NM, Bueno CR Jr, Secco M, Jazedje T, Costa Valadares M, Fussae Suzuki M, Bartolini P, Vainzof M, Zatz M. Preclinical studies with umbilical cord mesenchymal stromal cells in different animal models for muscular dystrophy. J Biomed Biotechnol 2011;DOI:10.1155/2011/715251.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7(2):211-228.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13(12):4279-4295.
Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res 2000;2(6):477-488.
AUTHOR BIO:
Michael Buratovich received his Ph.D. in Cell and Developmental Biology from UC Irvine in the laboratory of Peter Bryant where he worked on tumor suppressor genes in Drosophila melanogaster. He worked as a postdoctoral research fellow at Sussex University with Robert Whittle on the role of Wnt proteins in patterning the peripheral nervous system, and at University of Pennsylvania with Betsy Wilder on Wnt signaling during development. Since 1999, he has been a member of the faculty of Spring Arbor University (Spring Arbor, MI) in the Biochemistry department. He also served as a visiting scientist at Boston University in the laboratory of Joseph Ozer where he worked on the role of basal transcription factors in stem cell differentiation, and has collaborated with Amr Amin at the University of Al-Ain on cancer research. He is zealous about communicating science to the public and passionately blogs at http://www.beyondthedish.wordpress.com.