Chimeric Antigen Receptor T-cells or CAR T-cells are genetically engineered white blood cells that have been taken from a patient’s own blood, genetically engineered to express a receptor that can tightly bind to cancer cells, expanded in culture, and then re-administered into the patient’s blood. These CAR T-cells then act like guided anti-cancer missiles that find, attack, and kill cancer cells. The results of CAR T-cell treatments have been astoundingly successful, and the FDA has just approved several such treatments for specific cancers. On May 1, the Food and Drug Administration (FDA) approved the CAR T-cell therapy tisagenlecleucel (Kymriah) for adults with certain types of non-Hodgkin lymphoma. Last year, FDA approved another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), for the treatment of diffuse large B-cell lymphoma (DLBCL).
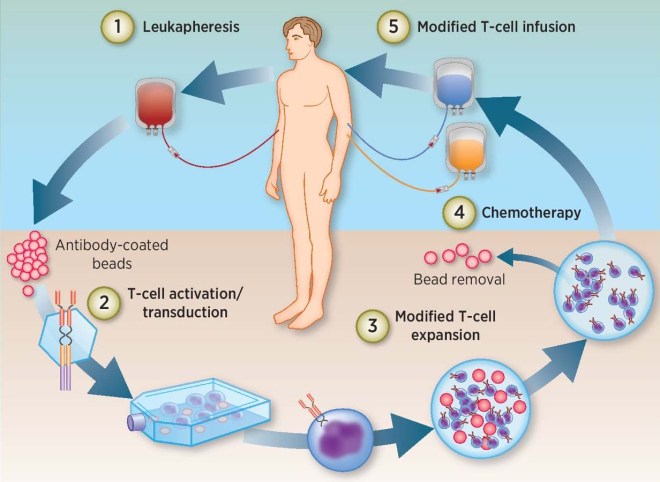
CAR-T cell therapies are effective against blood cancers like lymphoma and leukemia, but when it comes to solid tumors, this treatment has been less effective. Can CAR T-cell treatments be improved to attack solid tumors? Research teams at Memorial Sloan-Kettering Cancer Center (MSKCC) and Eureka Therapeutics have bet that they can. In a proof-of-concept study, MSKCC and Eureka Therapeutics scientists designed tumor-specific T-cells that express “checkpoint inhibitor” antibodies that protect the T-cell and allow it to evade the immunosuppressive tumor microenvironment found within a solid tumor. The results of this study were published in the journal Nature Biotechnology.
Checkpoint inhibitors are monoclonal antibodies bind to cell surface proteins on white blood cells that are used by cancer cells to disengage the white cells from the tumor. These checkpoint inhibitors, such as PD-1, when bound by cancer cells, cause the white blood cells to “forget” that they ever encountered the tumor. This effectively permits the tumor to hide from the immune system. Checkpoint inhibitors have successfully treated some solid tumors. In this experiment, the engineered CAR-T cells produced a single-variable fragment (scFv) PD-1 blocking antibody that is similar to already commercially available checkpoint inhibitor drugs. This checkpoint inhibitor antibody innocuously binds to PD-1 and prevents the cancer cell engaging it. This pulls back the tumor’s invisibility cloak and the CAR T cell recruits other neighboring immune cells to gang up on the tumor and kill it.
By using a mouse model, this study examined two different types of the anti-PD-1-expressing CAR-T cells; one of which targeted B-cell cancers and another that targeted solid tumors from in the ovary and the pancreas. ovarian and pancreatic cancer. These groups discovered that their anti-PD-1-expressing CAR-T cells stayed near the tumor site longer and, once inside the tumor, they recruited other neighboring tumor-fighting cells to wake up and sock it to the tumor.
This very exciting finding may be foundational for future targeted therapies. “We can build CAR-T cells to secrete a variety of different molecules, tailored to the needs of the patient,” he says. “It’s not just limited to this one drug,” said Renier Brentjens, Director of the Cellular Therapeutics Center at MSK and one of the pioneers of CAR therapy.