Eukaryotic organisms include every living thing with the exception of bacteria, Bacteria are known as prokaryotes, and they do not have an organized nucleus. Eukaryotic cells, on the other hand, have an organized nucleus in which that houses the chromosomes, which are linear molecules of DNA.
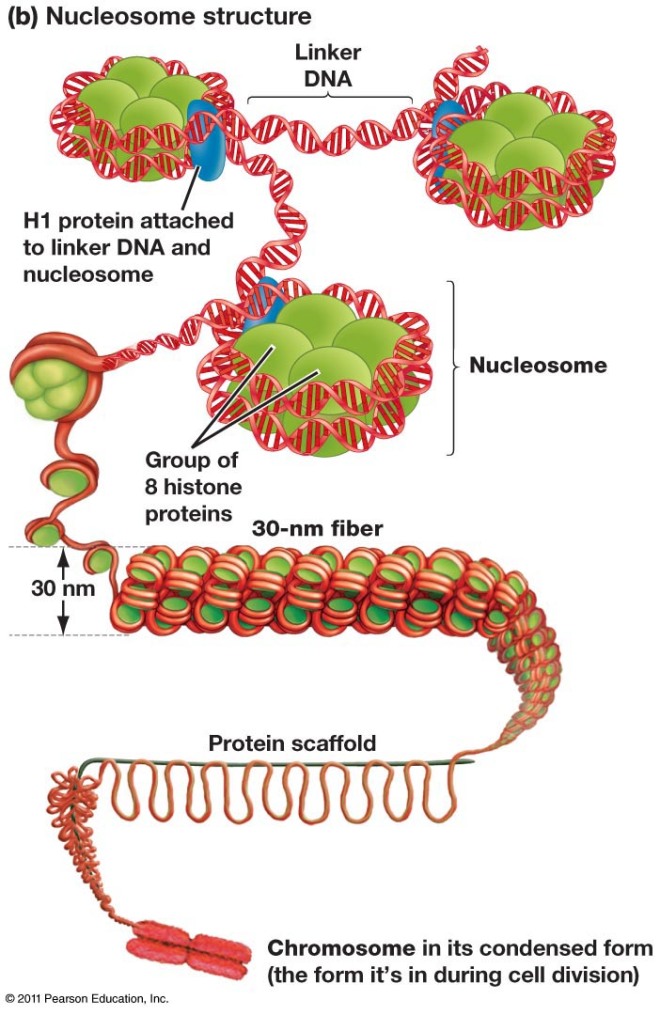
DNA is the molecule that stores genetic information. The chromosomes of eukaryotic organisms are sometimes rather long. How then does the cell manage to store all that DNA in such a small compartment such as the nucleus? The answer is that DNA in eukaryotic cells is wound into a tight configuration known as chromatin.
Chromatin consists of DNA molecules that are spooled around a cylindrical structure made of histone proteins. There are four so-called “core histones” that compose the cylinders and the DNA winds around these histone cores. Then a non-core histone called H1 pulls the histone cylinders with their DNA wound about them together to form higher-order structures. The histone cylinders wound about with DNA are called “nucleosomes” or “core particles.” The assembled clusters to nucleosomes are called “30 nanometer solenoids.”
You might think that DNA all wound into chromatin would be difficult to access and transcribe. If you think that, then you are correct. How then does the cell access DNA wound into chromatin? It modifies the histones so that the grip the histones have on the DNA is loosened. Since histones are positively charged and DNA is negatively charged (lots of phosphate), the two molecules bind to each other rather tightly. However, If histones are decorated with acetate groups, they become less positively charged and bind to DNA less tightly. This opens up the chromatin for gene expression. However, if histones are decorated with methyl groups (CH3), then proteins bind the histones and cinch the DNA even more tightly so that nothing is expressed. This is known as the “histone code,” since geneticists can use the chemical modifications of histones to make highly educated guesses about if genes will be expressed and the levels at which they will be expressed.
A research team at Stanford University in Palo Alto, CA, led my Thomas Rando, professor of neurological sciences and chief of the Veterans Affairs Palo Alto Health Care System’s Neurology service, has identified characteristic differences in histone modifications between stem cells from the muscles of young mice and old mice. Rando’s team also identified histone signatures characteristic of sleeping or quiescent and active stem cells in the muscles of young mice.
Rando said, “We’ve been trying to understand both how the different states a cell finds itself in can be defined by the markings on the histones surrounding its DNA, and to find an objective way to define the ‘age’ of a cell.”
All the cells of our body share the same genes, but these cells can be remarkably different in their function, structure, shape, and metabolism. Only a fraction of a cell’s genes are actually turned one and are actively making proteins. A muscle cells produced muscle-specific proteins and a liver cell makes liver cell specific proteins. Rando’s team has generated data that suggests that these same kinds of on/off differences may distinguish old stem cells from young stem cells.
First a little background in necessary. In 2005, Rando and others published a study that demonstrated that stem cells in several tissues from older mice, including muscle, seemed to act younger after continued exposure to the blood of a younger mouse. The capacity of these stem cells in older mice to divide, differentiate, and repopulate tissues declines with advancing age. However, after these stem cells from older mice were exposed to younger mouse’s blood, their ability to proliferate and repair tissues resembled those of their stem-cell counterparts in younger animals (see Conboy IM et al., Nature. 2005 433(7027):760-4).
Rando and his group asked the next question: “What is happening inside these cells that make them act as though they are younger?” The first place Rando and others decided to look was the chemical modifications of their histones. The cell population they examined was muscle satellite cells, which are relatively easy to isolate and grow in culture. Normally, muscle satellite cells sit within skeletal muscles and do well little. However, once the muscle is damaged, muscle satellite cells wake up, swing into action, and divide and fuse with damaged muscle fibers to repair them.
In mice that are old, histones in muscle satellite cells are a mixture of signals that tell expression to stop and signals that tell gene expression to go. However, in satellite cells from younger mice, the histones are largely a collection of go signals with only few stop signals. According to Rando, “Satellite cells can sit around for practically a lifetime in a quiescent state, not doing much of anything. But they’re ready to transform to an activated state as soon as they get the word that the tissue needs repair. So you might think that satellite cells would be already programmed in a way that commits them solely to the ‘mature muscle cell’ state.” Thus you would expect those genes specific for other tissues like skin, brain or fat would be marked with stop signals.
Instead quiescent satellite cells taken from the younger mice contained histones with a mixture of stop and go signals in those genes ordinarily reserved for other tissues. This was similar to what was observed in mature muscle-specific genes. Satellite cells from older mice were pockmarked with stop signals interspersed with go signals.
Are these changes typical of those that occur in other types of stem cells in other tissues? That is presently unknown. Also, what is the signal in the blood from the younger mice that causes the satellite cells function as though they are young?” Rando said, “We don’t have the answers yet. But now that we know what kinds of these changes occur as these cells age, we can ask which of these changes reverse themselves when an old cell goes back to becoming a young cell.”
Rando’s group is presently examining if the signatures they have identified in satellite cells generalize to other kinds of adult stem cells as well.