Scientists from Cambridge University have designed a new protocol that will convert pluripotent stem cells into primitive gut stem cells that have the capacity to differentiate into liver, pancreas, or some other gastrointestinal structure.
Nicholas Hannan and his colleagues at the University of Cambridge Welcome Trust MRC Stem Cell Institute have developed a technique that allows researchers to grow a pure, self-renewing population of stem cells that are specific to the human foregut, which is the upper section of the human digestive system. These types of stem cells are known as “foregut stem cells” and they can be used to make liver, pancreas, stomach, esophagus, or even parts of the small intestine. Making these types of gastrointestinal tissues can provide material for research into gastrointestinal abnormalities, but might also serve as a source of material to treat type 1 diabetes, liver disease, esophageal and stomach cancer, and other types of severe gastrointestinal diseases.
“We have developed a cell culture system which allows us to specifically isolate foregut stem cells in the lab,” said Hannan. “These cells have huge implications for regenerative medicine, because they are the precursors to the thyroid upper airways, lungs, liver, pancreas, stomach, and biliary systems.”
Hannan did this work in the laboratory of Ludovic Vallier, and they think that their technique will provide the means to analyze the precise embryonic development of the foregut in greater detail. “We now have a platform from which we can study the early patterning events that occur during human development to produce intestines, liver, lungs, and pancreas,” said Hannan.
To make foregut stem cells, Hannan begins with a pluripotent stem cell line; either an embryonic stem cell line or an induced pluripotent stem cell line. Then he differentiated them into definitive endoderm by treating them with CDM-PVA and activin-A (100 ng/ml), BMP4 (10 ng/ml), bFGF (20 ng/ml), and LY294002 (10 mM) for 3 days. Once they differentiated into endoderm, the endodermal cells were grown in RPMI+B27 medium with activin-A (50 ng/ml) for 3-4 days in order to generate foregut stem cells.
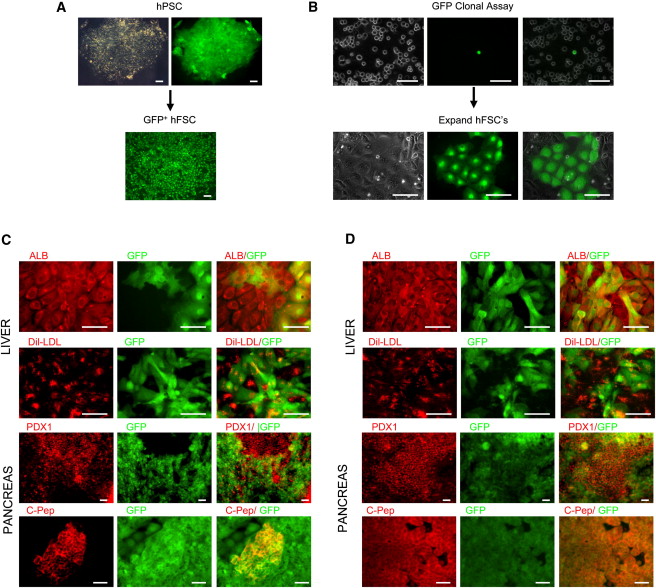
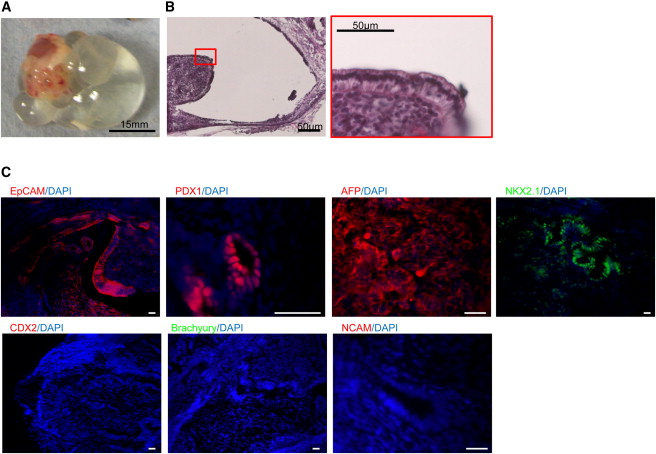
These foregut stem cells (FSCs) can self-renew, and can also differentiate into any part of the foregut. Thus, FSCs can grow robustly in culture, and they can also differentiate into foregut derivatives. However, these cells also do not form tumors. When injected into mice, they failed to form tumors.
What are the advantages to FSCs as opposed to making pancreatic cells or liver cells from pluripotent stem cells? These types of experiments always create cultures that are impure. Such cultures are difficult to use because not all the cells have the same growth requirements and they would be dangerous for therapeutic purposes because they might contain undifferentiated cells that might grow uncontrollably and cause a tumor. Therefore, FSCs provide a better starting point to make pure cultures of pancreatic tissues, liver tissues, stomach tissues and so on.
Ludovic Vallier, the senior author of this paper said this of his FSCs, “What we have now is a better starting point – a sustainable platform for producing liver and pancreatic cells. It will improve the quality of the cells that we produce and it will allow us to produce the large number of uncontaminated cells we need for the clinical applications of stem cell therapy.”
Vallier’s groups is presently examining the mechanisms that govern the differentiation of FSCs into specific gastrointestinal cell types in order to improve the production of these cells for regenerative medicine.