Producing dopamine-making neurons from stem cells for transplantation into Parkinson’s disease patients remains challenging. Differentiating stem cells into dopaminergic neurons is not as efficient a process as we would like it to be. While several laboratories have managed to make pretty good batches of dopaminergic neurons, reliably producing large and pure batches of dopamine-making neurons from pluripotent stem cells is still somewhat problematic. Secondly, transplanting dopamine-making neurons into either the midbrain or the striatum of the brain represents another patch of problems because the production of too much dopamine can cause unwanted, uncontrollable movements. Preclinical assessments of stem cell-derived dopamine neurons in laboratory animals have produced positive, but highly varied results, even though the transplanted cells are very similar at the time of transplantation.
“This has been frustrating and puzzling, and has significantly delayed the establishment of clinical cell production protocols,” said Malin Parmar, who led the study at Lund University.
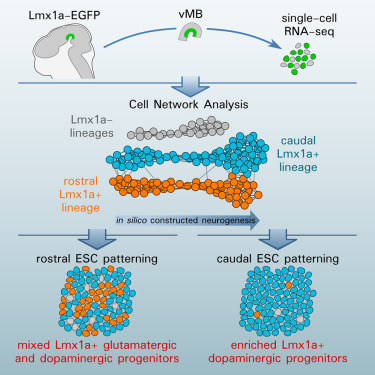
To address this issue, Parmar and his colleagues used modern global gene expression studies to gain a better understand the molecular changes that drive the differentiation of stem cells into dopamine-making neurons. Parmar conducted these experiments in collaboration with a team of scientists at Karolinska Institute. In their paper, which appeared in the journal Cell Stem Cell, Parmar and his colleagues used single-cell RNA seq to construct the neuronal development of dopaminergic neurons.
These neurons are characterized by the expression of a gene called LMX1a. However, it turns out that LMX1a-expressing neurons includes not only midbrain dopaminergic neurons (see below at the substantia nigra), but also subthalamic nuclear neurons.
These findings reveal that markers used to identify midbrain dopaminergic neurons do not specifically isolate midbrain dopaminergic neurons, but isolate a mixture of cells. Is there a way to separate these two populations?
Indeed, there is. Parmar and his colleagues in the laboratory of Thomas Perlmann showed that although dopaminergic neurons from the midbrain and subthalamic nuclear neurons are related, they do express a distinct profile of genes that are specific to the two cell types. The authors argue that the application of these distinct marker genes can help optimize those protocols that differentiate dopaminergic neurons from pluripotent stem cells.
See Nigel Kee and others, “Single-Cell Analysis Reveals a Close Relationship between Differentiating Dopamine and Subthalamic Nucleus Neuronal Lineages,” Cell Stem Cell, 2016; DOI: 10.1016/j.stem.2016.10.003.