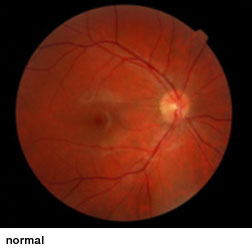
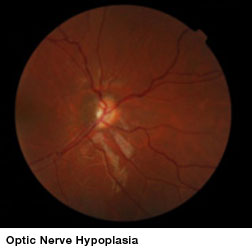
Optic nerve hypoplasia (ONH), an underdevelopment of optic nerves that occurs during fetal development, can appear as an isolated condition or as a part of a group of disorders characterized by brain anomalies, developmental delay, and endocrine abnormalities. ONH is a leading cause of blindness in children in North America and Europe and is the only cause of childhood blindness that shows increasing prevalence. No treatments have been shown to improve vision in these children.
Because stem cells heal or even regenerate some tissues, some have considered stem cell treatments as an option for this condition. However, a very small clinical study at Children’s Hospital Los Angeles found no evidence that stem cell therapies improve vision for children with optic nerve hypoplasia (ONH). Their results are reported in the Journal of the American Association for Pediatric Ophthalmology and Strabismus (AAPOS).
Families with a child that has ONH are traveling to China to undergo stem cell treatments that would be illegal in the United States. Because there are presently no viable treatment options available to improve vision in ONH children, such trips are often an act of desperation. The American Association for Pediatric Ophthalmology and Strabismus has also expressed its concern about these procedures, which are usually rather expensive, and have a dubious safety record.
Pediatric neuro-ophthalmologist Mark Borchert, MD, director of both the Eye Birth Defects and Eye Technology Institutes in The Vision Center at Children’s Hospital Los Angeles, realized that a controlled trial of sufficient size was needed to evaluate whether stem cell therapy is effective as a treatment for children with ONH. He agreed to conduct an independent study at the behest of Beike Biotech, which is based in Shenzhen, China and offers a stem cell treatment for ONH. This treatment uses donor umbilical cord stem cells and injects these cells into the cerebrospinal fluid.
Beike Biotech identified 10 children with bilateral ONH (ages 7 to 17 years) who had volunteered to travel to China for stem cell therapy. These patients gave their consent to participate in the study and Children’s Hospital found matched controls from their clinic. However, only two case-controlled pairs were evaluated because Beike Biotech was only able to recruit two patients.
Treatments consisted of six infusions over a 16-day period of umbilical cord-derived mesenchymal stem cells and daily infusions of growth factors. Visual acuity, optic nerve size, and sensitivity to light were to be evaluated one month before stem cell therapy and three and nine months after treatment.
Unfortunately no therapeutic effect was found in the two case-control pairs that were enrolled. “The results of this study show that children greater than 7 years of age with ONH may have spontaneous improvement in vision from one examination to the next. This improvement occurs equally in children regardless of whether or not they received treatment. Other aspects of the eye examination included pupil responses to light and optic nerve size; these did not change following treatment. The results of this research do not support the use of stem cells in the treatment of ONH at this time,” said lead author Cassandra Fink, MPH, program administrator at The Vision Center, Children’s Hospital Los Angeles.
However, confounding factors affect the interpretation of these results because the test subjects received additional alternative therapies (acupuncture, functional electrical stimulation and exercise) while receiving stem cell treatments. They were not supposed to receive such treatments. Additionally, the investigators could not determine the effect of these additional therapies on the subjects’ eyes.
“This study underscores the importance of scientifically testing these procedures to validate them and ensure their safety. Parents of afflicted children should be aware that the science behind the use of stem cell technology is unclear. This study takes a step toward testing this technology and finds no beneficial effect,” said William V. Good, MD, senior associate editor, Journal of AAPOS and Clinical Professor of Ophthalmology and Senior Scientist at the Smith-Kettlewell Eye Research Institute.
Basically, we have an incredibly small study that is also poorly controlled. Because the optic nerve forms during embryonic, fetal and postnatal development, using stem cells to make new nerves seems like a long shot as a treatment. I better treatment strategy might be to increase the myelination of the optic nerve with neural stem cells, oligodendrocyte precursor cells (OPCs), or Schwann cells. In general, this study does little to establish the lack of efficacy of such a stem cell treatment.