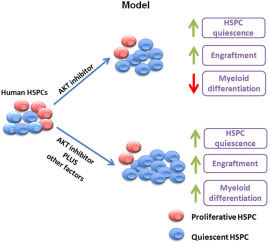
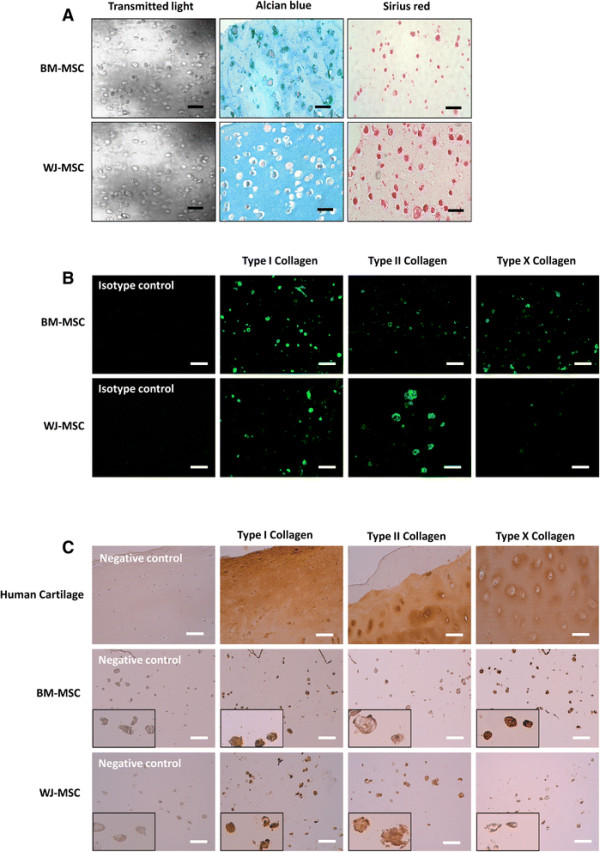
The umbilical cord connects the baby to the placenta and contains umbilical arteries, umbilical veins, and a gooey material between the umbilical vessels called Warton’s jelly. Warton’s Jelly (WJ), besides being rich in extracellular matrix molecules also contains a mesenchymal stem cell population that is rather primitive. These WJ mesenchymal stem cells or WJMSCs have excellent potential for therapeutic strategies.
Lian Gao and her colleagues from the Navy General Hospital in Beijing, China, in collaboration with coworkers from the Shenzhen Beike Cell Engineering Research Institute in Shenzhen, China conducted a clinical trial that examined the use of these WJMSCs in human patients who had suffered a heart attack. The results are as interesting as they are suggestive and were published in the journal BMC Medicine.
First we must consider the design of the study. Gao and others recruited 160 heart attack patients who were no younger than 18 and no older than 80-years old. All patients had to be free of liver or kidney disease, cancer or some other terminal illness. They were admitted to 11 hospitals in China between February 2011 and January 2012 and had suffered from a documented heart attack as defined by symptoms and their EKG (ST elevation). All patients has also been treated with the implantation of a stent within 12 hours of their heart attack and still retained a respectable amount of movement of the heart wall in the left ventricle. If patients were outside these parameters, they were excluded from the study.
Of the 160 patients who were recruited for the study, 44 were excluded, either because they did not fit within the exclusion criteria, did not wish to participate in the trial, or opted out for undisclosed reasons. This left 116 patients who were randomly assigned to the placebo group or the experimental group (58 in each group). Of these two groups, the placebo group had one patient discontinue the study because of a bout with stomach cancer. The experimental group had one patient die ten days into the trial, another was lost because they moved and a third patients withdrew because of leukemia. This left 57 subjects for the placebo group and 55 for the experiment group who went through all 18 months of follow-up after their respective procedures.
There were two end points for this clinical trial after patients were observed for 18 months after the procedure. The first was safety and this was measured by examining the number of adverse effects (AEs) within these 18 months. Such AEs include things like death, hospitalization for worsening heart function, severe arrhythmias, repeated coronary intervention, blood clots forming in the stents (stent thrombosis), coronary artery obstruction, and the growth of extra tissue in the heart that does not belong there, disorders of the immune system and so on. The second end pointy was efficacy of the implanted cells. To ascertain this, the function of the heart was measured using positron emission computer tomography (PET), and single-photo-emission computer tomography or SPECT. These imaging procedures allow cardiologists to take very precise snapshots of the heart and determine with a good deal of accuracy the performance of the heart.
The WJMSCs were acquired from umbilical cords that were donated from healthy mothers who had delivered healthy babies by means of Caesarian section. 21 of these umbilical cords had their blood vessels removed and then the gelatinous tissue surrounding the vessels was removed, sliced up, and cultured. The MSCs in the gelatinous tissue, which is Warton’s Jelly, migrated from the WJ to the culture dishes. After three passages in the culture dishes, he cells were harvested, concentrated, and tested for viruses, toxins, and cell viability. All cells were negative for viruses and toxins and other contaminants, and were also clearly MSCs, based on the ensemble of cell surface proteins that presented on their membranes, and showed high degrees of viability.
In infuse the cells into the hearts of the patients, six million WJMSCs were delivered into the coronary arteries using the usual over-the-wire techniques that are used to place stents, except that instead of placing stents, WJMSCs were slowly released into the coronary arteries. The cells will home to the damaged heart tissue and are able to pass through the blood vessels into the area of the infarct. Patients receiving the placebo, only received infusions of physiological saline solution, which was used to resuspend the WJMSCs.
The results are very encouraging. With respect to safety, the number of AEs was approximately the same for both groups. In the words of the study, “The groups did not differ in occurrences of MACEs (major adverse cardiac events), including death, recurrences of AMIs (acute myocardial infarctions) and re-hospitalization due to heart failure, during the course of treatment and the 18-month follow-up period.” There were no indications of cancer or the increase in tumor-specific molecules in the blood of the patients from either group. No biochemical or immune abnormalities were observed in any pf the patients either. The stomach cancer in one patient in the placebo group and leukemia in a patient from the experimental group were shown to be unrelated to the procedures. Therefore, at 18 months after the procedure, the infusion of these cells appears to be safe.
As to the efficacy of the procedure, there were significant improvements in the heart function of patients who had received the WJMSCs over those who had received placebo. First of all, the baseline heart function of patients in both groups was approximately the same on the average, except that the patients in the experimental group had slightly better heart parameter than those in the placebo group. Therefore, the efficacy of this procedure was determined by measuring the change in heart performance after the procedure. Patients who had received the placebo had about a 3% increase in the uptake of the F18-labeled sugar molecule after 4 months. The uptake of this marker indicates the presence of live cells. An increase in uptake of the modified sugar molecule shows that some new heart tissue has been produced, probably by the resident stem cell population in the heart. The experimental group, however, after 4 months showed an approximate 7% increase in PET signal intensity. This shows that a good deal more heart cells are being formed in the WJMSC-treated hearts that in the placebo-treated hearts. The SPECT imaging assays the “perfusion” of the heart tissue or the degree to which the heart tissue is being fed by blood vessels. After a heart attack, the dead area of the heart lacks blood vessels and its poor perfusion can affect nearby areas. The placebo-treated patients had a roughly 4% increase in SPECT signal, whereas the WJMSC-treated group had a 7% increase. Thus, the WJMSC-treated hearts had more blood vessels to feed the blood, oxygen and nutrients to the heart muscle and therefore, better perfusion.
Finally, the percentage of blood ejected by the heart during each contraction increase about 3% in the placebo group, but increase by about 8% in the WJMSC-treated group after 18 months. This parameter of heart function, the ejection fraction, is a very important measure of heart function and the fact that it significantly increased in the WJMSC-treated patients over the placebo-treated patients is an important finding.
This was a double-blinded, placebo-controlled study that determined the safety and efficacy of infusions of WJMSCs into the hearts of patients who had recently suffered from a heart attack. In animal experiments, these cells have been shown to increase heart function, increase blood vessel density in the hearts of animals, and increase resident heart-specific stem cell activity in the heart (see Lupu and others, Cell Physiol Biochem 2011; 28:63-76; Gao and others, Cell Transplant 2013; 22:1883-1900; Lopez Y, and others, Current Stem Cell Res Ther 2013;8:46-59). This clinical trial suggests that those benefits documented in laboratory animals might translate to human patients.
This is not a perfect study. These patients will need to be followed for several years to establish that these benefits are long-term and not short-term. Also, there is no indication that patients were given a 6-minute walking test to determine if the improvements in cardiac function translated to improvements in basic activities. However, it is an interesting study and it suggests that banking WJMSCs in addition to cord blood might be a good idea for use in trials like this one and maybe, someday for treatments of heart attack patients.