Huntington’s disease (HD) is a debilitating and invariably fatal disease that results from mutations in the IT15 gene. IT151 stands for “interesting transcript 15,” but it is more commonly referred to as the “huntingtin” gene. Mutations in the front of the gene (exon 1 for those who are interested) expand a run of CAG codons, and these mutations are probably the result of DNA polymerase slippage. Because CAG codons encode the amino acid glutamine, the mutant proteins contain long polyglutamine repeats and these repeats tend to clump inside neurons.
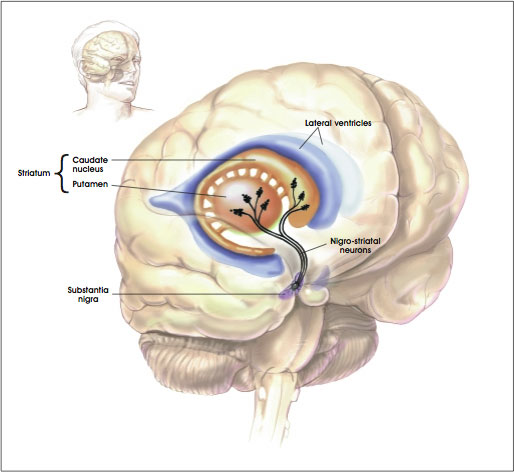
These protein aggregates form in neurons of the “striatum.” The striatum is a region of the brain that is also called the striate nucleus of the striate body. The striatum receives its name from the fact that it is organized in striped layers of gray and white matter. The striate nucleus is part of the cerebrum or forebrain.
Mutant Huntington (Htt) protein has a toxic that causes cell death by means of unknown mechanisms. Clinically, the most obvious symptoms of HD involve involuntary movements of the arms, legs, and face. But the severe cognitive and personality changes are the most devastating to HD patients and most troubling for their caregivers.
Researchers are using animal models of HD to study the disease pathogenesis, to elucidate areas of the brain involved in structural and functional decline, and to evaluate potential therapeutic interventions. These animal models include injecting toxins into the brain to kill off those populations of neurons that typically die in HD patients, and transgenic models in which animals are bred with either extra mutant copies of the Htt gene or a pair of copies of the mutant Htt gene that have replaced the original, normal copies. All of these model systems have limitations, but they are all useful in some way for assessing the pathology of HD.
This long introduction leads us to new data from the laboratory of Steve Goldman, the co-director of the University of Rochester Medical Center’s Center for Translational Medicine. Goldman and his colleagues triggered the production of new neurons in mice that had a rodent form of HD. These new neurons successfully integrated into the brain’s existing neural networks and dramatically extended the survival of the mice.
“This study demonstrates the feasibility of a completely new concept to treat Huntington’s disease, by recruiting the brain’s endogenous neural stem cells to regenerate cell lost the disease,” said Goldman.
One of the types of neurons most commonly affected in HD patients is the medium spinal neuron, which is critical to motor control. Goldman banked on findings from previous studies in his laboratory on canaries. Songbirds such as canaries have the ability to lay down new neurons in the adult brain when mating season comes. The male birds, in response to a flush of male sex hormones,, grow a gaggle of new neurons in the vocal control centers of the brain, and this provides the bird the means to sing specific songs in order to attract mates. This event is known as adult neurogenesis, and Goldman and Fernando Nottebohm of the Rockefeller University discovered this phenomenon in the early 1980s.
“Our work with canaries essentially provided us with the information we needed to understand how to add new neurons to adult brain tissue,” said Goldman. Once we mastered how this happened in birds, we set about how to replicate the process in the adult mammalian brain.”
Humans possess the ability to make new neurons, but Goldman’s lab demonstrated in the 1990s that a font of neuronal precursor cells exist in the lining of the ventricles (these are structures at the very center of the brain and spinal cord that are filled with cerebrospinal fluid). In early development, these cells are actively producing neurons.
Shortly after birth, the neural stem cells stop generating neurons and produce support cells called glia. Some parts of the human brain continue to produce neurons into adulthood, the most prominent example is the hippocampus, where memories are formed and stored. However, the striatum, new neuron production is switched off in adulthood.
Goldman sought to switch neuron production back on in the striatum. He tested a cadre of growth factors that would switch the neural stem cells of the striatum (a region that is ravaged by HD) from producing new glia to producing new neurons. Goldman, however, had some help from his recent work in canaries. Namely that once mating season was upon the birds, targeted expression of brain-derived neurotrophic factor (BDNF) flared up in the vocal centers of the brain, where many new neurons were being produced.
Goldman used genetically engineered viruses to express BDNF and another protein called “Noggin” in the striatum. Goldman and others found that a single intraventricular injection of the adenoviruses expressing BDNF and Noggin triggered the sustained recruitment of new neurons in both normal of R6/2 (HD) mice. These treated mice also showed that the newly formed neurons were recruited to form new medium spiny neurons; the ones destroyed in HD. These new neurons also matured and achieved circuit integration.
Also the treated mice showed delayed deterioration of motor function and substantially increased survival.
When the same experiments were conducted in squirrel monkeys, there was a similar addition of new striatal neurons.
Thus, induced neuronal addition may therefore represent a promising avenue for decreasing the ravages of HD and increasing cognitive ability.