The Minneapolis Heart Institute Foundation has announced a new clinical trial that will examine the ability of a stem cell combination to treat patients with ischemic heart failure.
In patients who have suffered from former heart attacks, clogged coronary blood vessels and heart muscle that hibernates can result in a heart that no longer works well enough to support the life of the patient. The lack of blood flow to vital parts of the heart and an increasing work load can result is so-called “Ischemic heart failure.” Such heart failure after a previous heart attack is one of the leading cause of death and morbidity in the world. According to the World Health Organization, ischemic heart disease affects more than 12% of the world’s population.
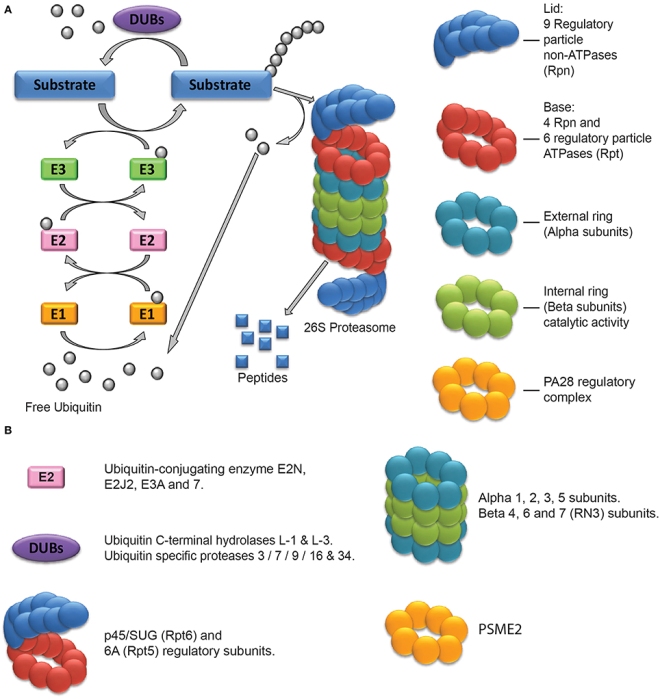
Stem cell therapy has been tested as a potential treatment for ischemic heart disease. Despite flashes of remarkable success, the overall efficacy of these treatments has been relatively modest. Most clinical trials have used the patient’s own bone marrow cells. In this case, the cell population is very mixed and it might not even be stem cell populations in the bone marrow that are eliciting recovery. Also, the quality of each patient’s bone marrow is probably quite varied, which makes standardizing such experiments remarkably difficult. Other clinical trials have used bone marrow derived mesenchymal cells [MSCs]. Several clinical trials with MSCs have seen some improvement in patients. MSCs seem to induce the formation of new blood vessels and also seem to induce endogenous stem cell populations in the heart to come to life and fix the heart. Other trials have used cardiac stem cells (CSCs) that were derived from biopsies of the heart. Even though fewer clinical trials have tested the efficacy of CSCs in human patients, the trials that have been conducted suggest that these cells can truly regenerate damaged heart tissue.
The Minneapolis Heart Institute Foundation® (MHIF) has announced a new clinical trial which will examine the combination of MSCs with CSCs to treatment patients with ischemic heart failure. This clinical trial, the CONCERT study, will be led by Principal Investigator Jay Traverse, MD. The CONCERT study will implant MSC’s and CSC’s in order to determine if the combination would be more successful than using either alone based on pre-clinical studies in swine demonstrating an enhanced synergistic effect of the combination.
CONCERT is sponsored by the National Institutes of Health and the Cardiovascular Cell Therapy Research Network (CCTRN), of which MHIF is a charter member. This will be a phase II clinical trial, which means that the focus of this leg of the study is to assess the relative safety of CSCs and MSCs, delivered either alone, or in combination, in comparison to placebo, and to measure the efficacy of the stem cell cocktail as well. To that end, researchers will measure and note any change or improvement in left ventricular (LV) function by cardiac MRI as well as changes in various clinical outcomes (survival, 6-minute walking, blood pressure, etc.), and quality of life.
This phase II study is a randomized, blinded, placebo-controlled study that will enroll 160 subjects at seven different CCTRN sites throughout the U.S. All recruited subjects will have ischemic cardiomyopathy and an ejection fraction 5%). This is significant, because some work in animals suggests that CSCs can make new heart muscle tissue that can shrink the heart scar. The first 16 patients were recently enrolled in a FDA-required safety run-in phase, but the remaining patients will be enrolled in the fall after a three-month safety analysis is performed. Incidentally, this is the first cardiac stem cell trial to perform MRIs on patients with defibrillators and pacemakers
“This combination of cells represents the most potent cell therapy product ever delivered to patients,” said Dr. Traverse. “Confirming that both types of stem cells together work better than either individual cell type could lead to improved patient outcomes and better quality of life for ischemic heart failure patients.”