Vicki Wheelock at the UC Davis Medical Center has registered clinical trial number NCT01937923, which is otherwise known as “PRE-CELL.” This clinical trial will use various imaging techniques, laboratory tests, and clinical evaluations of Huntington’s disease (HD) patients to map the disease progression over 12-18 months. This trial will then hopefully identify candidates for a new trial in which these patients will be implanted with mesenchymal stem cells that secrete nerve growth factors. This represents one of the first clinical trials to examine the use of mesenchymal stem cells in the treatment of HD
The rationale for this study comes from a 2012 study in mice. Ofer Sadan, Eldad Melamed, and Daniel Offen from the Rabin Medical Center in Tel Aviv University, Israel, used R6/2 mice to test the efficacy of nerve growth factor-secreting mesenchymal stem cells isolated from bone marrow . In this paper, Sadan and others isolated mesenchymal stem cells from the bone marrow of healthy human volunteers and mice and then cultured them in special growth media that induces these cells to secrete special nerve growth factors. These so-called NTF+ cells were then transplanted into the striatum of R6/2 mice.
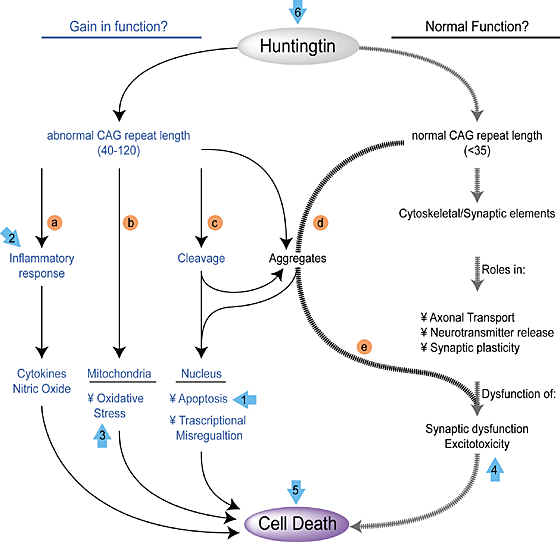
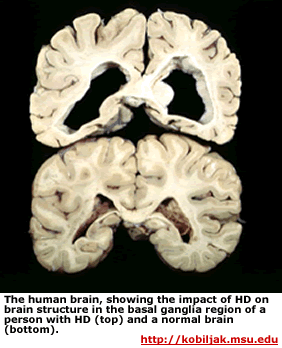
R6/2 mice express part of the human HTT gene; specifically the part that causes HD. Since HD is an inherited disease, there is a specific gene responsible for the vast majority of HD cases, and that gene is the human HTT gene, which encodes the Huntington protein. The function of the Huntington protein is uncertain, but it is found at high levels in neurons, even though it is found in other tissues as well, and dysfunctional Huntington protein affects neuron health.
The HTT gene in HD patients contains the insertion of extra copies of the CAG triplet. The more CAG triplets are inserted into the HTT gene, the more severe the HD caused by the mutation. The hitch is that normal copies of the HTT gene has multiple copies of this CAG repeat. CAG encodes the amino acid glutamine, and Huntington contains a stretch of glutamine residues that seem to allow the protein to interact with other proteins found in neurons. When this glutamine stretch becomes too long, the protein is toxic and it begins to kill the cells. How long is too long? Research has pretty clearly shown that people whose HTT genes contain less than 28 CAG virtually never develop HD. People with between 28–35 CAG repeats, are usually unaffected, but their children are at increased risk of developing HD. People whose HTT genes contain 36–40 CAG repeats may or may not show HD symptoms, and those who have over 40 copies almost always are afflicted with HD.
Now, back to R6/2 mice. These animals contain a part of the human HTT gene that has 150 CAG triplets. These mice show the characteristic cell death in the striatum and have behavioral deficits. In short R6/2 mice are pretty good model systems to study HD.
Sadan and others implanted MSCs that had been conditioned in culture to express high levels of nerve growth factors. Then these cells were transplanted into the striatum of R6/2 mice. R6/2 mice were also injected with buffer as a control.
The results showed that injections of NTF+ MSCs before the onset of symptoms did little good. The mice still showed cell death in the brains and behavioral deficits. However, NTF+ MSCs injected later (6.5 weeks), resulted in temporary improvement in the ability of the R6/2 mice to move and these cells also extended their life span. These results were published in the journal PLoS Currents (2012 Jul 10;4:e4f7f6dc013d4e).
Other work, also by Sadan and others, showed that injected MSCs tended to migrate to the damaged areas. When the injected cells were labeled with iron particles, they could be robustly observed with MRIs, and MRIs clearly showed that the injected cells migrated to the damaged areas in the brain (Stem Cells 2008; 26(10):2542-51). Another paper by Sadan and others also demonstrated that the striatum of NTF+ MSC-injected mice show less cell death than control mice (Sadan, et al. Exp Neurol. 2012; 234(2): 417-27). Other workers have also shown that implanted MSCs can provide improve symptoms in R6/2 mice and that they primary means by which they do this is by the secretion of nerve growth factors (Lee ST, et al. Ann Neurol 2009; 66(5): 671-81).
Thus, there is ample reason to suspect the PRECELL trial may lead to a stem cell-based clinical trial that will yield valuable clinical information. The animal data shows definite value in using preconditioned MSCs as a treatment for HD, and if the proper patients are identified by the PRE-CELL trials, then hopefully it will lead to a “CELL” trial in which HD patients are treated with NTF+ MSCs.
Mind you, this treatment will only delay HD at best and buy them time. Such treatments will not cure them. The NTF+ MSCs survive for a finite period of time in the hostile environment of the striatum of the HD patient, and the relief they will provide will be temporary. MSCs do not differentiate into neurons in this case, and they do not replace dead neurons, but they only help spare living neurons from suffering the same fate.
There is an MSC cell line that does make neurons, and if this cell line were used in combination with NTF+ MSCs, then perhaps neural replacement could be a possibility. Also neural precursor cells could be used in combination with NTF+ MSCs to increase their survival. Even then, as long as diseased neurons are producing toxic products, until gene therapy is perfected to the point that the actual genetic lesion in the striatal neurons is fixed, the deterioration of the striatum is inevitable. However, treatments like this could, potentially, delay this deterioration. This clinical trial should give us more information on exactly that question.
Two more points are worth mentioning. When fetal striatal grafts were implanted into the brains of HD patients, the grafts underwent disease-like degeneration, and actually made the patients worse (see Cicchetti et al. PNAS 2009; 106(30): 12483-8 and Cicchetti F, et al. Brain 2011; 134(pt 3): 641-52). Straight fetal implants do not seem to work. Please let’s put the kibosh on these gruesome experiments. Secondly, when neuronal precursor cells differentiated from human embryonic stem cells were implanted into HD rodents, the implanted cells formed some neurons and improved behavior to some extent, but non-neuronal differentiation remained a problem (Song J, et al., Neurosci Lett 2007; 423(1): 58-61). Having non-brain cells in your brain is a significant safety problem. Thus, embryonic stem cell-derived neuronal precursor cells do not seem to be the best bet to date either. So, this present clinical trial seems to be making the most of what is presently safely available.