According to two early-stage clinical trials reported in the journals Nature and Nature Medicine, a combination of two different antibodies against the Human Immunodeficiency Virus (HIV) have successfully diminished the virus levels in patients not taking anti-HIV medications. Infusions of these antibodies kept virus levels low in the blood of a percentage of these patients for two-three months, after which, blood quantities of HIV in these patients rebounded to their previous levels.
HIV is a member of a special class of viruses called “retroviruses.” Retroviruses receive their name from their ability to take an RNA copy of their genome and make a DNA copy of it. This process, called “reverse transcription,” is backwards from the way cells normally do things. In cells, genetic information is stored by DNA (deoxyribonucleic acid), and enzymes called RNA polymerases make an RNA copy of stretches of DNA. The process by which RNA copies are made from DNA is called “transcription.” In retroviruses, an enzyme called “reverse transcriptase” makes a double-stranded DNA copy of a single-stranded RNA genome. This transcriptional process reverses the direction of transcription; hence the name “retroviruses.”
Below is a picture of a HIV particle. The virus is surrounded by an envelope that is derived from the membranes of the host cell from which it budded. Inserted into those membranes are viral proteins that are decorated by sugars. These sugar-coated proteins are called “glycoproteins.” The HIV surface glycoproteins are called gp120, which means that the glycoprotein weighs 120 kilodaltons (a Dalton is the weight of a hydrogen atom and a kilodalton is the weight of 1,000 hydrogen atoms), and this viral glycoprotein is anchored to the viral membrane by means of another protein called gp41.
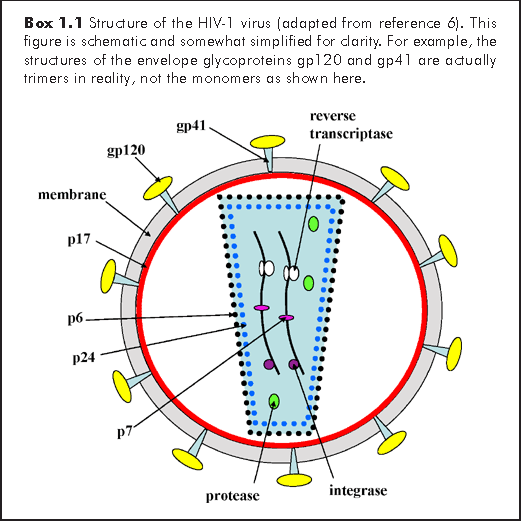
These two glycoproteins are the first thing that the acquired immune response responds to. Unfortunately, the viral reverse transcriptase is extremely error-prone and makes lots of mistakes as it copies its viral RNA genome into DNA copies. This means that when HIV infects cells, it produces progeny virus particles that vary tremendously. When these variant viruses particles are put under intense selective pressure, as in the case when the immune system makes antibodies against it or when the viruses is brushed back by means of highly active antiretroviral therapy (HAART), the variant viruses that survive the best (and show the highest levels of resistance to the treatment of immune system-based attack) will emerge as the dominant strain of virus in the infected person and as the treatment strategies change, so will the virus in adaptation to the latest attempts to eradicate it. It is an insidiously efficient mode of evading antiretroviral drugs and the immune system. HIV also infects and destroys T4 lymphocytes (T-helper lymphocytes), which are essential for activating B lymphocytes. Thus, HIV not only evades the immune response, but it permanently hamstrings it as well.
When a patient makes antibodies against the HIV surface glycoproteins, the virus simply changes the amino acid sequence of the protein and those neutralizing antibodies no longer prevent the virus from infecting cells. Therefore, scientists have worked very hard to make custom antibodies that neutralize the virus in such a way that would make it difficult for the virus to adapt.
Michel Nussenzweig, an immunologist at the Rockefeller University who led these studies and his colleagues, have made custom (“monoclonal” antibodies against specific pieces of gp120. Specifically, their custom antibodies target the base of the so-called “V3 loop” and the surrounding sugars. Past work shows these antibodies (3BNC117 and 10-1074) that target this one spot reduces virus levels in human patients infected with HIV (Caskey, M. and others, Nature 522, 487–491 (2015); Lynch, R. M. and others, Sci. Transl. Med. 7, 319ra206 (2015); and Caskey, M. and others, Nat. Med. 23, 185–191 (2017). Infusions of the antibody called 3BNC117 reduced virus levels of about 28 days in HIV-infected patients and 10-1074 reduced virus levels for one week in HIV-infected patients after which antibody-resistant viruses began to emerge. Nussenzweig and his group then had the novel idea of treatment patients with both antibodies and not just one of them. This way, the virus would have more hurdles to jump in order to adapt to this treatment.
In one of the clinical trials, 15 HIV-infected patients who had stopped taking all antiretroviral medications received three infusions of both monoclonal antibodies. Of these 15 patients, nine of them showed a significant reduction in viral load for 15 – 30 weeks, after which, virus levels in the blood rebounded.
In the second clinical trial, seven HIV-infected patients who had never taken antiretroviral drugs were given infusions of the two monoclonal antibodies. Initially, patients received infusions of 30 mg kg−1 of each of the antibodies and these infusions were very well tolerated. In those four patients whose HIV viral profile showed sensitivity to the dual antibody treatment in laboratory tests, this therapy reduced HIV-1 viral load by two factors of ten. This viral load reduction continued for three months following the first of up to three infusions. Additionally, and this is potentially the most exciting part of this study, none of these individuals developed resistance to both antibodies. According to The Scientist, the viral loads of these patients rebounded; it is possible that these patients stopped receiving the antibody treatments (I only have access to the abstract of the Nature Medicine article). However, the fact that the sensitive viruses stayed susceptible to the dual antibody treatment is quite encouraging.
Antibody treatments are diabolically expensive because making hybridoma cell lines that create the monoclonal antibodies is ridiculously labor- and resource-intensive. For this reason, Nussenzweig said, “Ultimately, this may not be good for everybody, and it’s expensive.”
When HIV-infected patients are placed on HAART, they must take those drugs daily, for the rest of their lives. These drugs have some potentially serious side effects, but they do tend to reduce progression of HIC infection to full-blown AIDS and reduce HIV-induced mortality (Kitahata, MM and others (2009), New England Journal of Medicine. 360 (18): 1815–1826; Lundgren, JD and others (2015), New England Journal of Medicine. 373 (9): 795–807; Danel, Christine and others (2015), The New England Journal of Medicine. 373 (9): 808–822). Neutralizing antibodies against the virus may provide longer-lasting treatments, but, to date, HIV quickly adapts to experimental antibody treatments (for example, see Trkola, A. and others, Nature Medicine 11, 615–622 (2005).
“Antibodies can be used as a safe, new treatment to allow people to go ‘off’ of drugs for several months,” Nancy Haigwood, an AIDS researcher at Oregon Health & Science University who was not part of these studies.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said that this work represents an “early step” toward antibody-based HIV interventions.
Both these studies are small, and only included a few patients. What we can glean from there, therefore, is limited. However, there are some definite bright spots from them. First of all, the dual antibody treatment was well tolerated in these patients. This suggests that antibody treatments might be rather safe. Again, larger studies are needed, but this is a potential bright spot of this study. Secondly, the dual antibody treatment did significantly reduce viral loads for some time in a percentage of both groups of patients. Thus, if the right antibody combination is found, it could potentially do some real damage to HIV levels in patients with susceptible viral populations. Third, the antibody-based treatment strategy might actually work in some cases.
The caveats are many. One is the cost. Such mass treatments of HIV patients around the world will prove horribly expensive and third-world countries with the largest HIV load will simply not be able to sustain such costs. Secondly, how long can such treatments give relief to patients when the virus simply adapts after a few weeks or months. Therefore, feasibility of antibody-based treatments are a second problem. Once potential use for antibody-based treatments that occurs to me are for those patients whose toxicity from HAART is just too onerous and the antibody-based treatments can give them a drug holiday to heal. Such patients would need to be pre-screened to ensure that their virus population is susceptible to the antibody treatment before starting it. Such HAART holidays may also cause a shift in their viral population to strains that are more susceptible to the HAART regimen they are taking. Therefore, such drug holidays/dual antibody treatments might (a big might) have knock-off benefits.
In any case, this early, preliminary data is hopeful, but it is just the beginning if antibody-based treatments are to make it to the clinic.