Huntington’s disease (HD) is an inherited disorder of the central nervous system characterized by progressive dementia, involuntary movements, and emotional deterioration. The brain is affected in patients with HD and the part of the brain that takes the biggest beating is the “neostriatum.”
The term “neostriatum” is almost certainly not a word that you hear terribly often in conversation. Therefore I will try to explain what it is. The outer layers of the brain are known as the cerebral cortex and they are composed of so-called “grey matter.” The cerebral cortex consists of grey matter because it is loaded with cells known as neurons. Beneath the cortical layer of the brain, lies a whole host of extensions of these neurons that reside in the cerebral cortex. These extensions are called “axons,” and beneath the cerebral cortex lies white matter, which consists, largely, of bundles of axons. Think of the neurons are plugs and the axons as extension cords. The neurons are plugged into each other by means of extension cords that extend from the cerebral cortex.
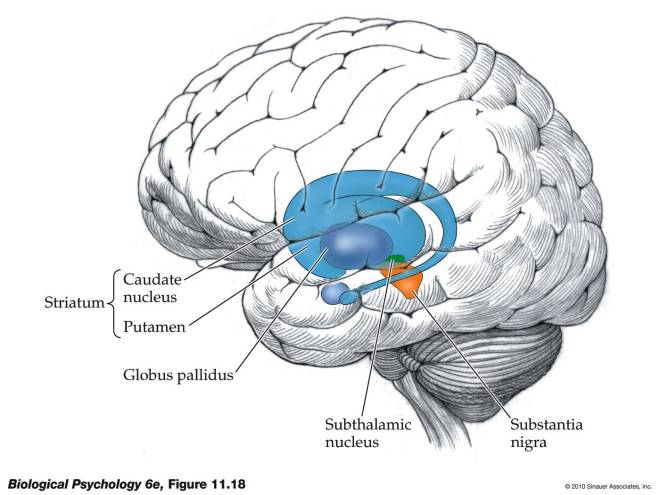
Now the cerebral cortex is not the only game in town. There are also clusters of neurons that lie beneath the cerebral cortex called “nuclei.” One of these nuclei beneath the cerebral cortex plays an extremely important role in voluntary motion, and this structure is called the “basal ganglia.” Here’s a picture to make things a little clearer:
As you can see in the figure, the striatum consists of two structures: the putamen and the caudate nucleus. The striatum or striate nucleus receives neural inputs from the cerebral cortex and inputs this neural information to the basal ganglia.
From a functional perspective, the striatum helps coordinate motivation with body movement. It facilitates and balances motivation with both higher-level and lower-level functions – for example, inhibiting one’s behavior in a complex social interaction and fine-motor functions involved in inhibiting small voluntary movement.
Some of the neural outputs from the striatum are excitatory – they stimulate other neurons. Other signals are inhibitory – they prevent the neurons to which they are connected from becoming stimulated. Inhibitory neurons release a chemical called “GABA.” These GABA-using neurons are very important for the work of the striatum, and it is exactly these neurons that die off at the greatest rate in patients with HD. Therefore, treatments for patients with HD have focused on replacing or protecting these GABA-using neurons.
Experimentally, you can induce an HD-like disease in rodents if you inject a chemical into their brains called quinolinic acid. Quinolinic acid causes many of the GABA-using neurons in the striatum to pack up and die, and for this reason, this chemical is heavily used in the laboratories of scientists who study HD and HD treatments.
In a paper by Marcus McLeod and others who did their work in the laboratory of Ivar Mendez, who was at Dalhousie University in Nova Scotia, Canada, but has since moved to the University of Saskatchewan, GABA-using neurons were made from cultured human neural precursor cells (hNPCs) and then implanted into the brains of rats that had been injected with quinolinic acid. The results were spectacularly successful. This work was published in the journal Cell Transplantation.
A definite twist with this particular paper is the way the GABA-using neurons were grown in culture; they were grown in bioreactors. Bioreactors are devices that support biological cells, processes, or organisms. They keep the environment of the cells constant, and provide a far superior way to grow cells or tissues in the laboratory. McLeod and his colleagues used human neural progenitor cells and grew them to large numbers in bioreactors. These expanded hNPCs were then differentiated them into GABA-using neurons and then injected into the brains of rats who has been treated with quinolinic acid.
The rat model allows the scientist to inject only one side of the brain with quinolinic acid. This leaves the intact side of the brain as a control tissue that can be compared with the injected one. The injected rats showed the characteristic death of the GABA-using neurons and the behavioral features that result from the death of these neurons. Such animals do not walk normally when they are led through a cylinder, and they have trouble finding their way through a maze. The animals that received the transplantations of the GABA-using neurons, however, performed almost as well in these tests as normal rats; not quite as well, but almost as well. The rats treated with quinolinic acid did quite poorly, as expected.
Upon post-mortem examination, the rats transplanted with GABA-using neurons shows a host of new GABA-using neurons in their striatums. These cells also underwent further maturation after transplantation, and they also made connections with other neurons.
Now this paper shows that the injected cells not only survived the transplantations, but they also matured, made connections and promoted recovery of many of the behavioral symptoms of HD. This procedure certainly has promise.
Having said all that, there are two caveats to these experiments. The rodent model is a good model as far as it goes, but it seems clear that the actual human disease turns the environment of the brain into a very inhospitable place. Transplanted cells in the case of human HD patients do not usually survive terribly well. It seems to me that treatments like this must be coupled with other treatments that seek to improve the actual cerebral environment. The second caveat to this experiment is that the neural progenitor cells were taken 10-week-old from aborted fetuses. While these scientists did not perform the abortions that ended the lives of these babies, it is more than little troubling that this research was done using the corpses of those babies whose lives were prematurely ended.
Nevertheless, despite these caveats, this paper represents a definite advance in the regenerative strategies available to treat HD patients.